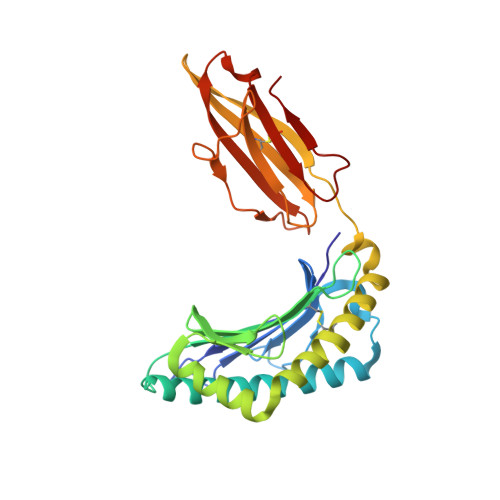
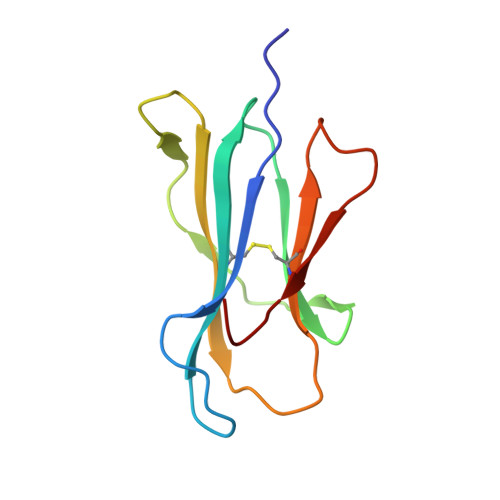
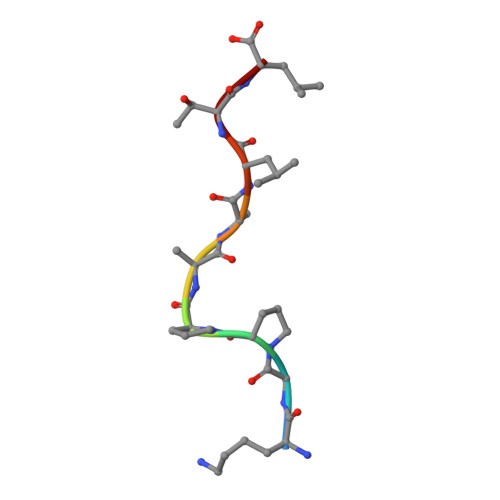
The structure and stability of the monomorphic HLA-G are influenced by the nature of the bound peptide
Walpole, N.G., Kjer-Nielsen, L., Kostenko, L., McCluskey, J., Brooks, A.G., Rossjohn, J., Clements, C.S.(2010) J Mol Biology 397: 467-480
- PubMed: 20122941
- DOI: https://doi.org/10.1016/j.jmb.2010.01.052
- Primary Citation of Related Structures:
3KYN, 3KYO - PubMed Abstract:
The highly polymorphic major histocompatibility complex class Ia (MHC-Ia) molecules present a broad array of peptides to the clonotypically diverse alphabeta T-cell receptors. In contrast, MHC-Ib molecules exhibit limited polymorphism and bind a more restricted peptide repertoire, in keeping with their major role in innate immunity. Nevertheless, some MHC-Ib molecules do play a role in adaptive immunity. While human leukocyte antigen E (HLA-E), the MHC-Ib molecule, binds a very restricted repertoire of peptides, the peptide binding preferences of HLA-G, the class Ib molecule, are less stringent, although the basis by which HLA-G can bind various peptides is unclear. To investigate how HLA-G can accommodate different peptides, we compared the structure of HLA-G bound to three naturally abundant self-peptides (RIIPRHLQL, KGPPAALTL and KLPQAFYIL) and their thermal stabilities. The conformation of HLA-G(KGPPAALTL) was very similar to that of the HLA-G(RIIPRHLQL) structure. However, the structure of HLA-G(KLPQAFYIL) not only differed in the conformation of the bound peptide but also caused a small shift in the alpha2 helix of HLA-G. Furthermore, the relative stability of HLA-G was observed to be dependent on the nature of the bound peptide. These peptide-dependent effects on the substructure of the monomorphic HLA-G are likely to impact on its recognition by receptors of both innate and adaptive immune systems.
- The Protein Crystallography Unit, Department of Biochemistry and Molecular Biology, School of Biomedical Sciences, Monash University, Clayton, Victoria 3800, Australia.
Organizational Affiliation: