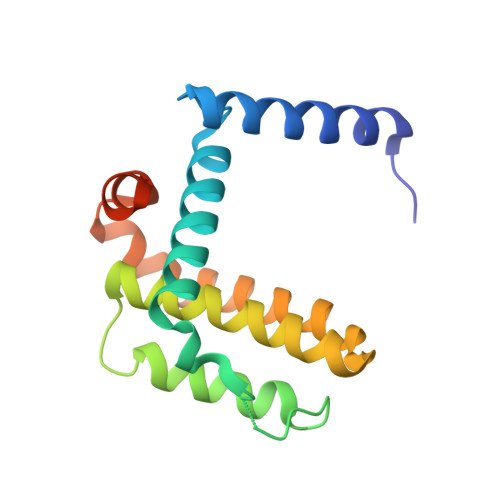
Conformational changes in Bcl-2 pro-survival proteins determine their capacity to bind ligands.
Lee, E.F., Czabotar, P.E., Yang, H., Sleebs, B.E., Lessene, G., Colman, P.M., Smith, B.J., Fairlie, W.D.(2009) J Biological Chem 284: 30508-30517
- PubMed: 19726685
- DOI: https://doi.org/10.1074/jbc.M109.040725
- Primary Citation of Related Structures:
3INQ, 3IO8, 3IO9 - PubMed Abstract:
Antagonists of anti-apoptotic Bcl-2 family members hold promise as cancer therapeutics. Apoptosis is triggered when a peptide containing a BH3 motif or a small molecule BH3 peptidomimetic, such as ABT 737, binds to the relevant Bcl-2 family members. ABT-737 is an antagonist of Bcl-2, Bcl-x(L), and Bcl-w but not of Mcl-1. Here we describe new structures of mutant BH3 peptides bound to Bcl-x(L) and Mcl-1. These structures suggested a rationale for the failure of ABT-737 to bind Mcl-1, but a designed variant of ABT-737 failed to acquire binding affinity for Mcl-1. Rather, it was selective for Bcl-x(L), a result attributable in part to significant backbone refolding and movements of helical segments in its ligand binding site. To date there are few reported crystal structures of organic ligands in complex with their pro-survival protein targets. Our structure of this new organic ligand provided insights into the structural transitions that occur within the BH3 binding groove, highlighting significant differences in the structural properties of members of the Bcl-2 pro-survival protein family. Such differences are likely to influence and be important in the quest for compounds capable of selectively antagonizing the different family members.
- The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria 3052, Australia.
Organizational Affiliation: