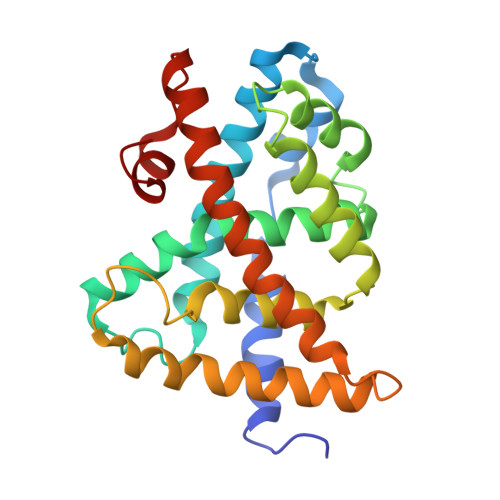
FXR agonist activity of conformationally constrained analogs of GW 4064.
Akwabi-Ameyaw, A., Bass, J.Y., Caldwell, R.D., Caravella, J.A., Chen, L., Creech, K.L., Deaton, D.N., Madauss, K.P., Marr, H.B., McFadyen, R.B., Miller, A.B., Navas, F., Parks, D.J., Spearing, P.K., Todd, D., Williams, S.P., Bruce Wisely, G.(2009) Bioorg Med Chem Lett 19: 4733-4739
- PubMed: 19586769
- DOI: https://doi.org/10.1016/j.bmcl.2009.06.062
- Primary Citation of Related Structures:
3HC5, 3HC6 - PubMed Abstract:
Two series of conformationally constrained analogs of the FXR agonist GW 4064 1 were prepared. Replacement of the metabolically labile stilbene with either benzothiophene or naphthalene rings led to the identification of potent full agonists 2a and 2g.
- Department of Medicinal Chemistry, GlaxoSmithKline, Research Triangle Park, NC 27709, USA.
Organizational Affiliation: