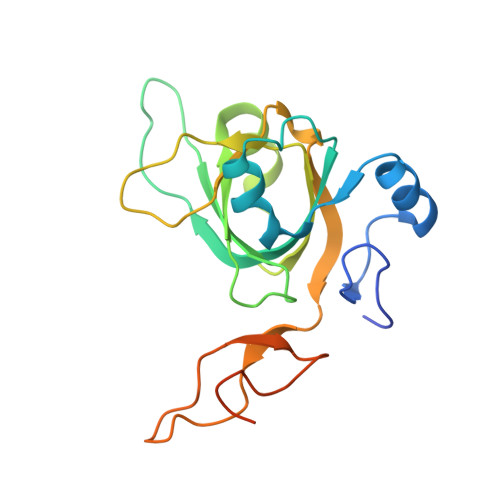
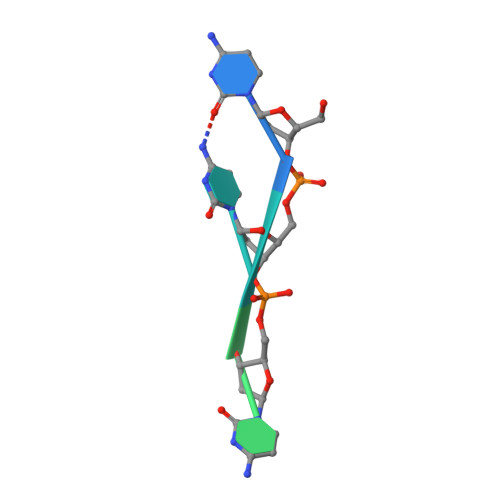
Physical Interactions between Mcm10, DNA, and DNA Polymerase {alpha}.
Warren, E.M., Huang, H., Fanning, E., Chazin, W.J., Eichman, B.F.(2009) J Biological Chem 284: 24662-24672
- PubMed: 19608746
- DOI: https://doi.org/10.1074/jbc.M109.020438
- Primary Citation of Related Structures:
3H15 - PubMed Abstract:
Mcm10 is an essential eukaryotic protein required for the initiation and elongation phases of chromosomal replication. Specifically, Mcm10 is required for the association of several replication proteins, including DNA polymerase alpha (pol alpha), with chromatin. We showed previously that the internal (ID) and C-terminal (CTD) domains of Mcm10 physically interact with both single-stranded (ss) DNA and the catalytic p180 subunit of pol alpha. However, the mechanism by which Mcm10 interacts with pol alpha on and off DNA is unclear. As a first step toward understanding the structural details for these critical intermolecular interactions, x-ray crystallography and NMR spectroscopy were used to map the binary interfaces between Mcm10-ID, ssDNA, and p180. The crystal structure of an Mcm10-ID*ssDNA complex confirmed and extended our previous evidence that ssDNA binds within the oligonucleotide/oligosaccharide binding-fold cleft of Mcm10-ID. We show using NMR chemical shift perturbation and fluorescence spectroscopy that p180 also binds to the OB-fold and that ssDNA and p180 compete for binding to this motif. In addition, we map a minimal Mcm10 binding site on p180 to a small region within the p180 N-terminal domain (residues 286-310). These findings, together with data for DNA and p180 binding to an Mcm10 construct that contains both the ID and CTD, provide the first mechanistic insight into how Mcm10 might use a handoff mechanism to load and stabilize pol alpha within the replication fork.
- Department of Biological Sciences, Vanderbilt University, Nashville, Tennessee 37232, USA.
Organizational Affiliation: