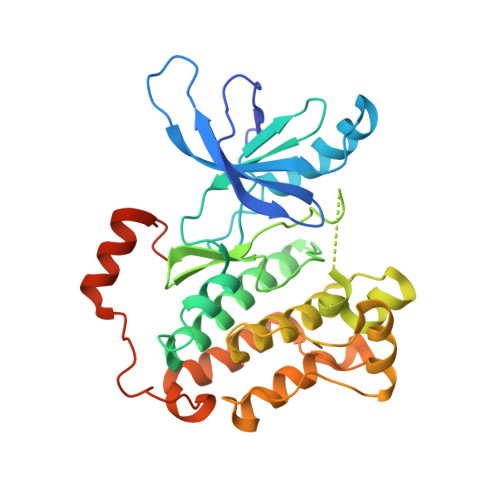
Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment.
Jura, N., Endres, N.F., Engel, K., Deindl, S., Das, R., Lamers, M.H., Wemmer, D.E., Zhang, X., Kuriyan, J.(2009) Cell 137: 1293-1307
- PubMed: 19563760
- DOI: https://doi.org/10.1016/j.cell.2009.04.025
- Primary Citation of Related Structures:
3GT8 - PubMed Abstract:
Signaling by the epidermal growth factor receptor requires an allosteric interaction between the kinase domains of two receptors, whereby one activates the other. We show that the intracellular juxtamembrane segment of the receptor, known to potentiate kinase activity, is able to dimerize the kinase domains. The C-terminal half of the juxtamembrane segment latches the activated kinase domain to the activator, and the N-terminal half of this segment further potentiates dimerization, most likely by forming an antiparallel helical dimer that engages the transmembrane helices of the activated receptor. Our data are consistent with a mechanism in which the extracellular domains block the intrinsic ability of the transmembrane and cytoplasmic domains to dimerize and activate, with ligand binding releasing this block. The formation of the activating juxtamembrane latch is prevented by the C-terminal tails in a structure of an inactive kinase domain dimer, suggesting how alternative dimers can prevent ligand-independent activation.
- Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, CA 94720, USA.
Organizational Affiliation: