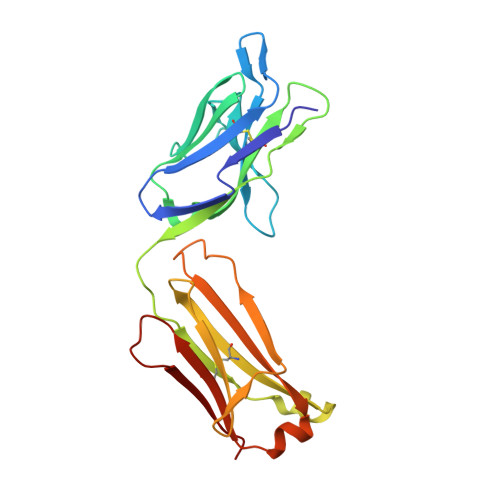
Crystal structure and thermodynamic analysis of diagnostic mAb 106.3 complexed with BNP 5-13 (C10A).
Longenecker, K.L., Ruan, Q., Fry, E.H., Saldana, S.C., Brophy, S.E., Richardson, P.L., Tetin, S.Y.(2009) Proteins 76: 536-547
- PubMed: 19274732
- DOI: https://doi.org/10.1002/prot.22366
- Primary Citation of Related Structures:
3E8U - PubMed Abstract:
B-type natriuretic peptide (BNP) is a naturally secreted regulatory hormone that influences blood pressure and vascular water retention in human physiology. The plasma BNP concentration is a clinically recognized biomarker for various cardiovascular diseases. Quantitative detection of BNP can be achieved in immunoassays using the high-affinity monoclonal IgG1 antibody 106.3, which binds an epitope spanning residues 5-13 of the mature bioactive peptide. To understand the structural basis of this molecular recognition, we crystallized the Fab fragment complexed with the peptide epitope and determined the three-dimensional structure by X-ray diffraction to 2.1 A resolution. The structure reveals the detailed interactions that five of the complementarity-determining regions make with the partially folded peptide. Thermodynamic measurements using fluorescence spectroscopy suggest that the interaction is enthalpy driven, with an overall change in free energy of binding, DeltaG = -54 kJ/mol, at room temperature. The parameters are interpreted on the basis of the structural information. The kinetics of binding suggest a diffusion-limited mechanism, whereby the peptide easily adopts a bound conformation upon interaction with the antibody. Moreover, comparative analysis with alanine-scanning results of the epitope explains the basis of selectivity for BNP over other related natriuretic peptides.
- Advanced Technology, Global Pharmaceutical Research and Development, Abbott Park, Illinois 60064, USA.
Organizational Affiliation: