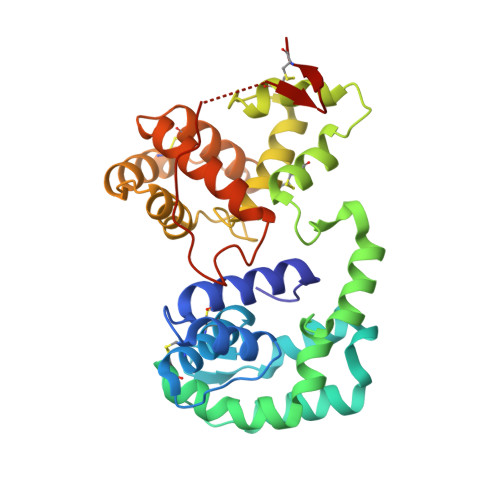
Multifunctionality and mechanism of ligand binding in a mosquito antiinflammatory protein
Calvo, E., Mans, B.J., Ribeiro, J.M., Andersen, J.F.(2009) Proc Natl Acad Sci U S A 106: 3728-3733
- PubMed: 19234127
- DOI: https://doi.org/10.1073/pnas.0813190106
- Primary Citation of Related Structures:
3DXL, 3DY9, 3DYE, 3DZT - PubMed Abstract:
The mosquito D7 salivary proteins are encoded by a multigene family related to the arthropod odorant-binding protein (OBP) superfamily. Forms having either one or two OBP domains are found in mosquito saliva. Four single-domain and one two-domain D7 proteins from Anopheles gambiae and Aedes aegypti (AeD7), respectively, were shown to bind biogenic amines with high affinity and with a stoichiometry of one ligand per protein molecule. Sequence comparisons indicated that only the C-terminal domain of AeD7 is homologous to the single-domain proteins from A. gambiae, suggesting that the N-terminal domain may bind a different class of ligands. Here, we describe the 3D structure of AeD7 and examine the ligand-binding characteristics of the N- and C-terminal domains. Isothermal titration calorimetry and ligand complex crystal structures show that the N-terminal domain binds cysteinyl leukotrienes (cysLTs) with high affinities (50-60 nM) whereas the C-terminal domain binds biogenic amines. The lipid chain of the cysLT binds in a hydrophobic pocket of the N-terminal domain, whereas binding of norepinephrine leads to an ordering of the C-terminal portion of the C-terminal domain into an alpha-helix that, along with rotations of Arg-176 and Glu-268 side chains, acts to bury the bound ligand.
- Laboratory of Malaria and Vector Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD 20852, USA.
Organizational Affiliation: