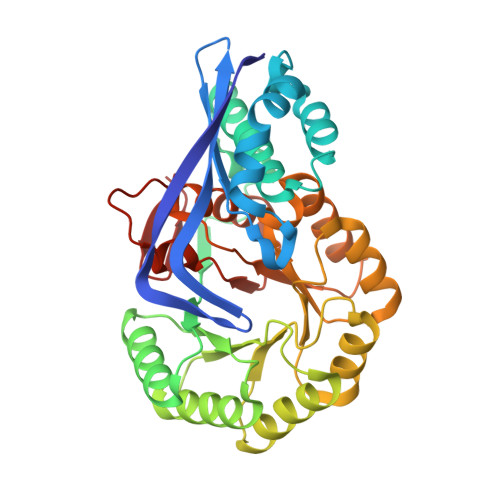
Discovery of a dipeptide epimerase enzymatic function guided by homology modeling and virtual screening.
Kalyanaraman, C., Imker, H.J., Fedorov, A.A., Fedorov, E.V., Glasner, M.E., Babbitt, P.C., Almo, S.C., Gerlt, J.A., Jacobson, M.P.(2008) Structure 16: 1668-1677
- PubMed: 19000819
- DOI: https://doi.org/10.1016/j.str.2008.08.015
- Primary Citation of Related Structures:
3DEQ, 3DER, 3DES, 3DFY - PubMed Abstract:
We have developed a computational approach to aid the assignment of enzymatic function for uncharacterized proteins that uses homology modeling to predict the structure of the binding site and in silico docking to identify potential substrates. We apply this method to proteins in the functionally diverse enolase superfamily that are homologous to the characterized L-Ala-D/L-Glu epimerase from Bacillus subtilis. In particular, a protein from Thermotoga martima was predicted to have different substrate specificity, which suggests that it has a different, but as yet unknown, biological function. This prediction was experimentally confirmed, resulting in the assignment of epimerase activity for L-Ala-D/L-Phe, L-Ala-D/L-Tyr, and L-Ala-D/L-His, whereas the enzyme is annotated incorrectly in GenBank as muconate cycloisomerase. Subsequently, crystal structures of the enzyme were determined in complex with three substrates, showing close agreement with the computational models and revealing the structural basis for the observed substrate selectivity.
- Department of Pharmaceutical Chemistry, School of Pharmacy, University of California, 600 16(th) Street, San Francisco, CA 94158, USA.
Organizational Affiliation: