Crystal structure of the uncharacterized human protein C8orf32 with bound peptide.
Bitto, E., Bingman, C.A., McCoy, J.G., Wesenberg, G.E., Phillips Jr., G.N.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
A newer entry is available that reflects an alternative modeling of the original data: 4W79
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Uncharacterized protein C8orf32 | 205 | Homo sapiens | Mutation(s): 4 Gene Names: C8orf32 EC: 3.5.1.122 | 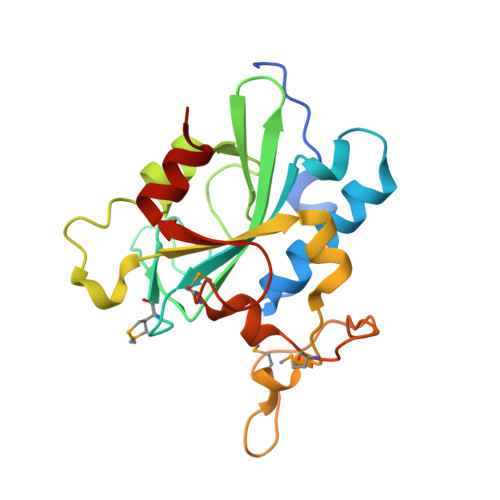 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q96HA8 (Homo sapiens) Explore Q96HA8 Go to UniProtKB: Q96HA8 | |||||
PHAROS: Q96HA8 GTEx: ENSG00000156795 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q96HA8 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Synthetic peptide | B [auth L] | 3 | N/A | Mutation(s): 0 |  |
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | C [auth A], D [auth A], E [auth A], F [auth A], G [auth A] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| EDO Query on EDO | I [auth A], J [auth A], K [auth A], L [auth A], M [auth A] | 1,2-ETHANEDIOL C2 H6 O2 LYCAIKOWRPUZTN-UHFFFAOYSA-N |  | ||
| CO3 Query on CO3 | H [auth A] | CARBONATE ION C O3 BVKZGUZCCUSVTD-UHFFFAOYSA-L |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| MSE Query on MSE | A | L-PEPTIDE LINKING | C5 H11 N O2 Se |  | MET |
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 34.322 | α = 90 |
| b = 64.039 | β = 90 |
| c = 113.66 | γ = 90 |
| Software Name | Purpose |
|---|---|
| DENZO | data reduction |
| SCALEPACK | data scaling |
| SHARP | phasing |
| DM | phasing |
| REFMAC | refinement |
| PDB_EXTRACT | data extraction |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |
| SHELXL-97 | phasing |