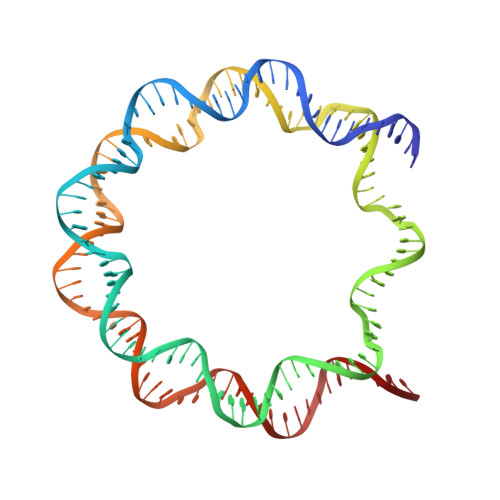
Structures of human nucleosomes containing major histone H3 variants
Tachiwana, H., Osakabe, A., Shiga, T., Miya, Y., Kimura, H., Kagawa, W., Kurumizaka, H.(2011) Acta Crystallogr D Biol Crystallogr 67: 578-583
- PubMed: 21636898
- DOI: https://doi.org/10.1107/S0907444911014818
- Primary Citation of Related Structures:
3AV1, 3AV2 - PubMed Abstract:
The nucleosome is the fundamental repeating unit of chromatin, via which genomic DNA is packaged into the nucleus in eukaryotes. In the nucleosome, two copies of each core histone, H2A, H2B, H3 and H4, form a histone octamer which wraps 146 base pairs of DNA around itself. All of the core histones except for histone H4 have nonallelic isoforms called histone variants. In humans, eight histone H3 variants, H3.1, H3.2, H3.3, H3T, H3.5, H3.X, H3.Y and CENP-A, have been reported to date. Previous studies have suggested that histone H3 variants possess distinct functions in the formation of specific chromosome regions and/or in the regulation of transcription and replication. H3.1, H3.2 and H3.3 are the most abundant H3 variants. Here, crystal structures of human nucleosomes containing either H3.2 or H3.3 have been solved. The structures were essentially the same as that of the H3.1 nucleosome. Since the amino-acid residues specific for H3.2 and H3.3 are located on the accessible surface of the H3/H4 tetramer, they may be potential interaction sites for H3.2- and H3.3-specific chaperones.
- Laboratory of Structural Biology, Graduate School of Advanced Science and Engineering, Waseda University, Tokyo 162-8480, Japan.
Organizational Affiliation: