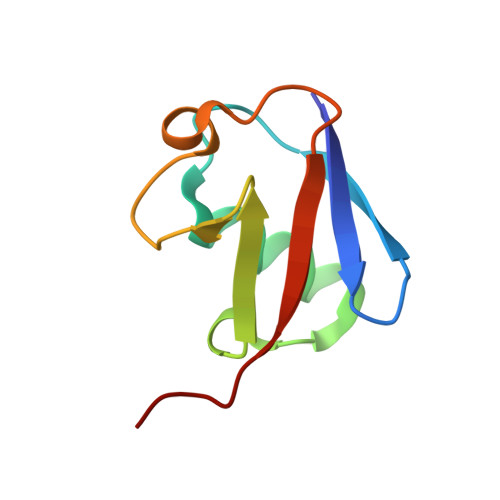
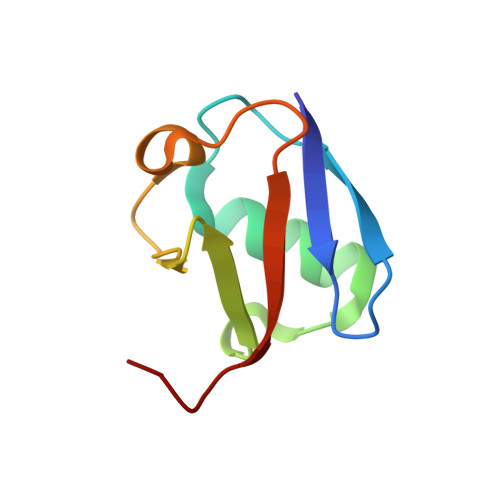
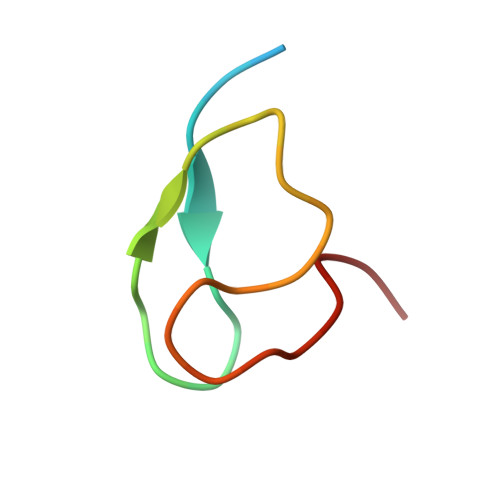
Structural basis for specific recognition of Lys 63-linked polyubiquitin chains by NZF domains of TAB2 and TAB3
Sato, Y., Yoshikawa, A., Yamashita, M., Yamagata, A., Fukai, S.(2009) EMBO J 28: 3903-3909
- PubMed: 19927120
- DOI: https://doi.org/10.1038/emboj.2009.345
- Primary Citation of Related Structures:
3A9J, 3A9K - PubMed Abstract:
TAB2 and TAB3 activate the Jun N-terminal kinase and nuclear factor-kappaB pathways through the specific recognition of Lys 63-linked polyubiquitin chains by its Npl4 zinc-finger (NZF) domain. Here we report crystal structures of the TAB2 and TAB3 NZF domains in complex with Lys 63-linked diubiquitin at 1.18 and 1.40 A resolutions, respectively. Both NZF domains bind to the distal ubiquitin through a conserved Thr-Phe dipeptide that has been shown to be important for the interaction of the NZF domain of Npl4 with monoubiquitin. In contrast, a surface specific to TAB2 and TAB3 binds the proximal ubiquitin. Both the distal and proximal binding sites of the TAB2 and TAB3 NZF domains recognize the Ile 44-centred hydrophobic patch on ubiquitin but do not interact with the Lys 63-linked isopeptide bond. Mutagenesis experiments show that both binding sites are required to enable binding of Lys 63-linked diubiquitin. We therefore propose a mechanism for the recognition of Lys 63-linked polyubiquitin chains by TAB2 and TAB3 NZF domains in which diubiquitin units are specifically recognized by a single NZF domain.
- Structural Biology Laboratory, Life Science Division, Synchrotron Radiation Research Organization, Institute of Molecular and Cellular Biosciences, The University of Tokyo, Tokyo, Japan.
Organizational Affiliation: