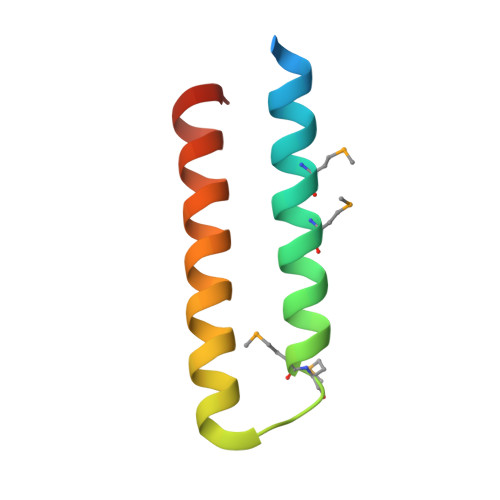
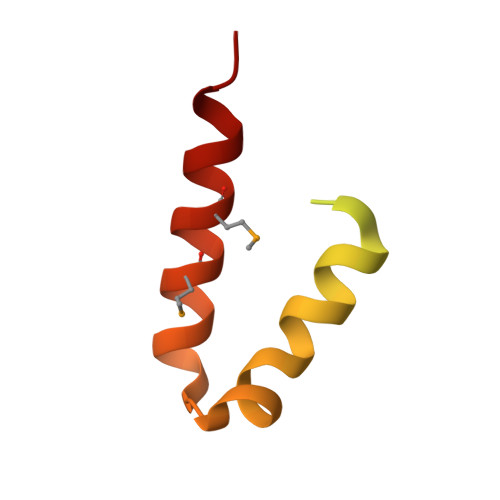
Structural characterization of the Yersinia pestis type III secretion system needle protein YscF in complex with its heterodimeric chaperone YscE/YscG.
Sun, P., Tropea, J.E., Austin, B.P., Cherry, S., Waugh, D.S.(2008) J Mol Biology 377: 819-830
- PubMed: 18281060
- DOI: https://doi.org/10.1016/j.jmb.2007.12.067
- Primary Citation of Related Structures:
2P58 - PubMed Abstract:
The plague-causing bacterium Yersinia pestis utilizes a type III secretion system to deliver effector proteins into mammalian cells where they interfere with signal transduction pathways that mediate phagocytosis and the inflammatory response. Effector proteins are injected through a hollow needle structure composed of the protein YscF. YscG and YscE act as "chaperones" to prevent premature polymerization of YscF in the cytosol of the bacterium prior to assembly of the needle. Here, we report the crystal structure of the YscEFG protein complex at 1.8 A resolution. Overall, the structure is similar to that of the analogous PscEFG complex from the Pseudomonas aeruginosa type III secretion system, but there are noteworthy differences. The structure confirms that, like PscG, YscG is a member of the tetratricopeptide repeat family of proteins. YscG binds tightly to the C-terminal half of YscF, implying that it is this region of YscF that controls its polymerization into the needle structure. YscE interacts with the N-terminal tetratricopeptide repeat motif of YscG but makes very little direct contact with YscF. Its function may be to stabilize the structure of YscG and/or to participate in recruiting the complex to the secretion apparatus. No electron density could be observed for the 49 N-terminal residues of YscF. This and additional evidence suggest that the N-terminus of YscF is disordered in the complex with YscE and YscG. As expected, conserved residues in the C-terminal half of YscF mediate important intra- and intermolecular interactions in the complex. Moreover, the phenotypes of some previously characterized mutations in the C-terminal half of YscF can be rationalized in terms of the structure of the heterotrimeric YscEFG complex.
- Macromolecular Crystallography Laboratory, Center for Cancer Research, National Cancer Institute at Frederick, P.O. Box B, Frederick, MD, USA.
Organizational Affiliation: