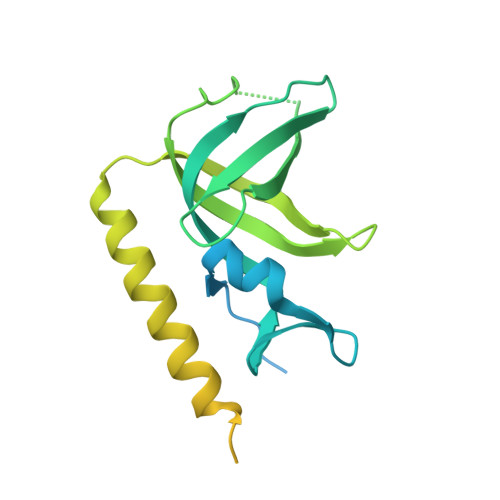
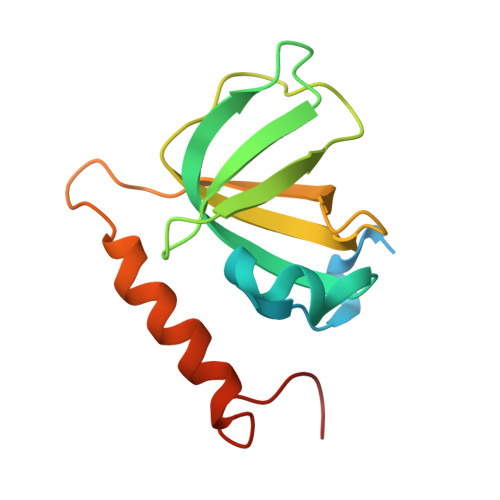
Structure of the Full-length Human RPA14/32 Complex Gives Insights into the Mechanism of DNA Binding and Complex Formation
Deng, X., Habel, J.E., Kabaleeswaran, V., Snell, E.H., Wold, M.S., Borgstahl, G.E.(2007) J Mol Biology 374: 865-876
- PubMed: 17976647
- DOI: https://doi.org/10.1016/j.jmb.2007.09.074
- Primary Citation of Related Structures:
2PI2, 2PQA, 2Z6K - PubMed Abstract:
Replication protein A (RPA) is the ubiquitous, eukaryotic single-stranded DNA (ssDNA) binding protein and is essential for DNA replication, recombination, and repair. Here, crystal structures of the soluble RPA heterodimer, composed of the RPA14 and RPA32 subunits, have been determined for the full-length protein in multiple crystal forms. In all crystals, the electron density for the N-terminal (residues 1-42) and C-terminal (residues 175-270) regions of RPA32 is weak and of poor quality indicating that these regions are disordered and/or assume multiple positions in the crystals. Hence, the RPA32 N terminus, that is hyperphosphorylated in a cell-cycle-dependent manner and in response to DNA damaging agents, appears to be inherently disordered in the unphosphorylated state. The C-terminal, winged helix-loop-helix, protein-protein interaction domain adopts several conformations perhaps to facilitate its interaction with various proteins. Although the ordered regions of RPA14/32 resemble the previously solved protease-resistant core crystal structure, the quaternary structures between the heterodimers are quite different. Thus, the four-helix bundle quaternary assembly noted in the original core structure is unlikely to be related to the quaternary structure of the intact heterotrimer. An organic ligand binding site between subunits RPA14 and RPA32 was identified to bind dioxane. Comparison of the ssDNA binding surfaces of RPA70 with RPA14/32 showed that the lower affinity of RPA14/32 can be attributed to a shallower binding crevice with reduced positive electrostatic charge.
- The Eppley Institute for Research in Cancer and Allied Diseases, 987696 Nebraska Medical Center, Omaha, NE 68198-7696, USA.
Organizational Affiliation: