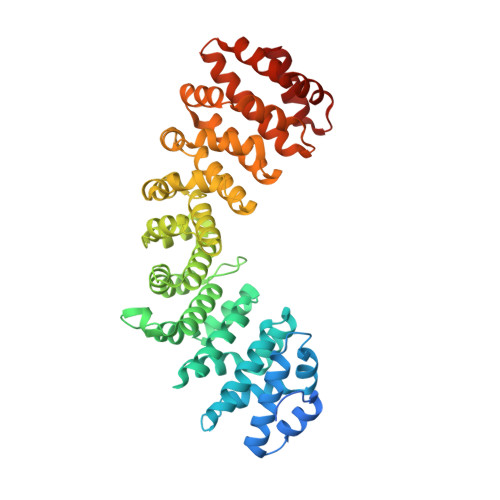
Crystal Structure of Rice Importin-Alpha and Structural Basis of its Interaction with Plant-Specific Nuclear Localization Signals.
Chang, C.-W., Counago, R.L.M., Williams, S.J., Boden, M., Kobe, B.(2012) Plant Cell 24: 5074
- PubMed: 23250448
- DOI: https://doi.org/10.1105/tpc.112.104422
- Primary Citation of Related Structures:
2YNR, 2YNS, 4B8J, 4B8O, 4B8P, 4BA3 - PubMed Abstract:
In the classical nucleocytoplasmic import pathway, nuclear localization signals (NLSs) in cargo proteins are recognized by the import receptor importin-α. Importin-α has two separate NLS binding sites (the major and the minor site), both of which recognize positively charged amino acid clusters in NLSs. Little is known about the molecular basis of the unique features of the classical nuclear import pathway in plants. We determined the crystal structure of rice (Oryza sativa) importin-α1a at 2-Å resolution. The structure reveals that the autoinhibitory mechanism mediated by the importin-β binding domain of importin-α operates in plants, with NLS-mimicking sequences binding to both minor and major NLS binding sites. Consistent with yeast and mammalian proteins, rice importin-α binds the prototypical NLS from simian virus 40 large T-antigen preferentially at the major NLS binding site. We show that two NLSs, previously described as plant specific, bind to and are functional with plant, mammalian, and yeast importin-α proteins but interact with rice importin-α more strongly. The crystal structures of their complexes with rice importin-α show that they bind to the minor NLS binding site. By contrast, the crystal structures of their complexes with mouse (Mus musculus) importin-α show preferential binding to the major NLS binding site. Our results reveal the molecular basis of a number of features of the classical nuclear transport pathway specific to plants.
- School of Chemistry and Molecular Biosciences and Institute for Molecular Bioscience, University of Queensland, Brisbane Qld 4072, Australia.
Organizational Affiliation: