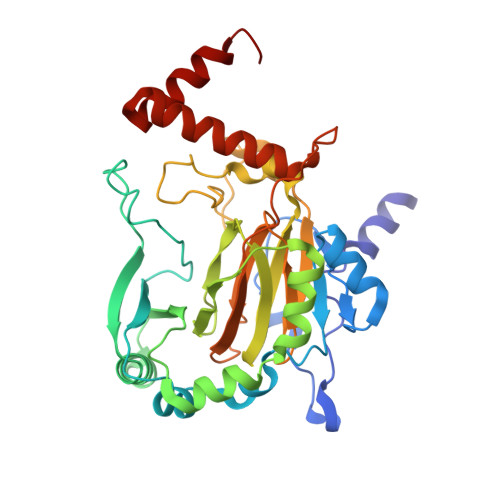
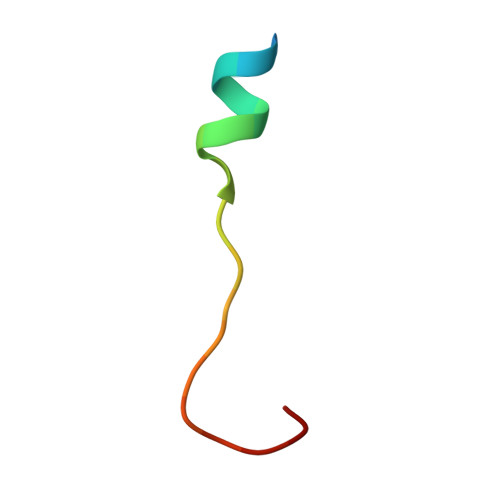
Asparagine and aspartate hydroxylation of the cytoskeletal ankyrin family is catalyzed by factor-inhibiting hypoxia-inducible factor.
Yang, M., Ge, W., Chowdhury, R., Claridge, T.D., Kramer, H.B., Schmierer, B., McDonough, M.A., Gong, L., Kessler, B.M., Ratcliffe, P.J., Coleman, M.L., Schofield, C.J.(2011) J Biological Chem 286: 7648-7660
- PubMed: 21177872
- DOI: https://doi.org/10.1074/jbc.M110.193540
- Primary Citation of Related Structures:
2XUM - PubMed Abstract:
Factor-inhibiting hypoxia-inducible factor (FIH) catalyzes the β-hydroxylation of an asparagine residue in the C-terminal transcriptional activation domain of the hypoxia inducible factor (HIF), a modification that negatively regulates HIF transcriptional activity. FIH also catalyzes the hydroxylation of highly conserved Asn residues within the ubiquitous ankyrin repeat domain (ARD)-containing proteins. Hydroxylation has been shown to stabilize localized regions of the ARD fold in the case of a three-repeat consensus ankyrin protein, but this phenomenon has not been demonstrated for the extensive naturally occurring ARDs. Here we report that the cytoskeletal ankyrin family are substrates for FIH-catalyzed hydroxylations. We show that the ARD of ankyrinR is multiply hydroxylated by FIH both in vitro and in endogenous proteins purified from human and mouse erythrocytes. Hydroxylation of the D34 region of ankyrinR ARD (ankyrin repeats 13-24) increases its conformational stability and leads to a reduction in its interaction with the cytoplasmic domain of band 3 (CDB3), demonstrating the potential for FIH-catalyzed hydroxylation to modulate protein-protein interactions. Unexpectedly we found that aspartate residues in ankyrinR and ankyrinB are hydroxylated and that FIH-catalyzed aspartate hydroxylation also occurs in other naturally occurring AR sequences. The crystal structure of an FIH variant in complex with an Asp-substrate peptide together with NMR analyses of the hydroxylation product identifies the 3S regio- and stereoselectivity of the FIH-catalyzed Asp hydroxylation, revealing a previously unprecedented posttranslational modification.
- Chemistry Research Laboratory and Oxford Centre for Integrative Systems Biology, University of Oxford, Oxford OX1 3TA, United Kingdom.
Organizational Affiliation: