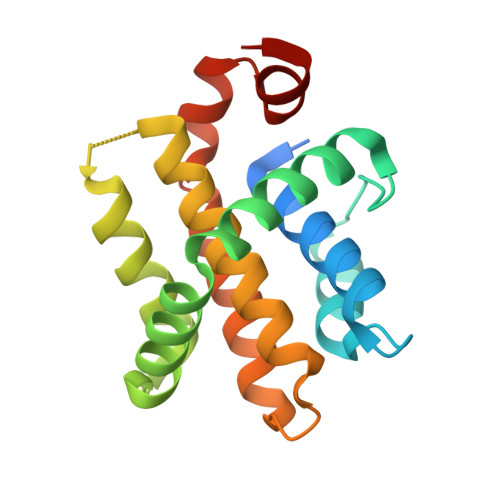
Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus Bhrf1.
Kvansakul, M., Wei, A.H., Fletcher, J.I., Willis, S.N., Chen, L., Roberts, A.W., Huang, D.C.S., Colman, P.M.(2010) PLoS Pathog 6: 1236
- PubMed: 21203485
- DOI: https://doi.org/10.1371/journal.ppat.1001236
- Primary Citation of Related Structures:
2V6Q, 2WH6, 2XPX - PubMed Abstract:
Epstein-Barr virus (EBV) is associated with human malignancies, especially those affecting the B cell compartment such as Burkitt lymphoma. The virally encoded homolog of the mammalian pro-survival protein Bcl-2, BHRF1 contributes to viral infectivity and lymphomagenesis. In addition to the pro-apoptotic BH3-only protein Bim, its key target in lymphoid cells, BHRF1 also binds a selective sub-set of pro-apoptotic proteins (Bid, Puma, Bak) expressed by host cells. A consequence of BHRF1 expression is marked resistance to a range of cytotoxic agents and in particular, we show that its expression renders a mouse model of Burkitt lymphoma untreatable. As current small organic antagonists of Bcl-2 do not target BHRF1, the structures of it in complex with Bim or Bak shown here will be useful to guide efforts to target BHRF1 in EBV-associated malignancies, which are usually associated with poor clinical outcomes.
- The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria, Australia.
Organizational Affiliation: