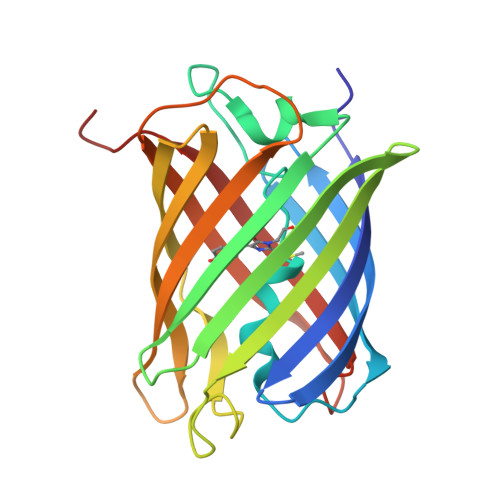
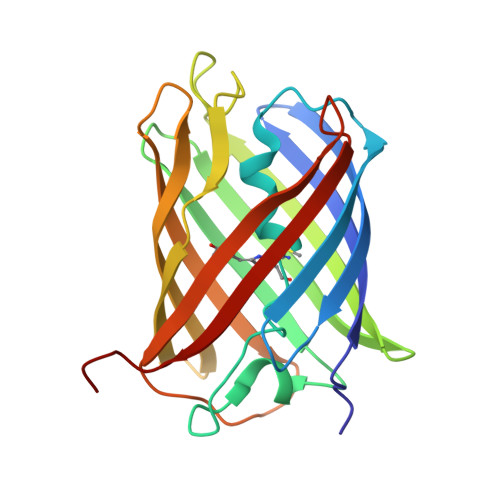
A Noncytotoxic Dsred Variant for Whole-Cell Labeling.
Strack, R.L., Strongin, D.E., Bhattacharyya, D., Tao, W., Berman, A., Broxmeyer, H.E., Keenan, R.J., Glick, B.S.(2008) Nat Methods 5: 955
- PubMed: 18953349
- DOI: https://doi.org/10.1038/nmeth.1264
- Primary Citation of Related Structures:
2V4E - PubMed Abstract:
A common application of fluorescent proteins is to label whole cells, but many RFPs are cytotoxic when used with standard high-level expression systems. We engineered a rapidly maturing tetrameric fluorescent protein called DsRed-Express2 that has minimal cytotoxicity. DsRed-Express2 exhibits strong and stable expression in bacterial and mammalian cells, and it outperforms other available RFPs with regard to photostability and phototoxicity.
- Department of Biochemistry and Molecular Biology, The University of Chicago, Gordon Center W238, Chicago, Illinois 60637, USA.
Organizational Affiliation: