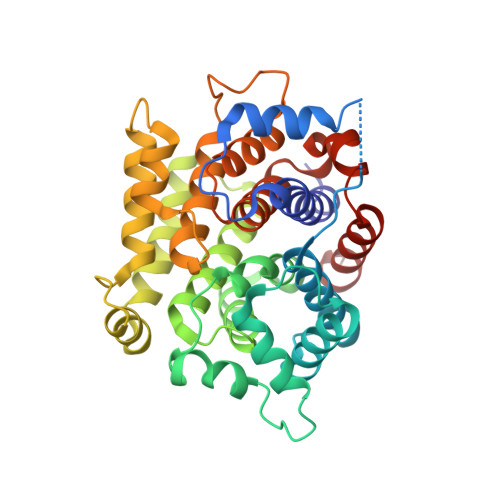
Structure of mouse ADP-ribosylhydrolase 3 (mARH3).
Mueller-Dieckmann, C., Kernstock, S., Mueller-Dieckmann, J., Weiss, M.S., Koch-Nolte, F.(2008) Acta Crystallogr Sect F Struct Biol Cryst Commun 64: 156-162
- PubMed: 18323597
- DOI: https://doi.org/10.1107/S1744309108001413
- Primary Citation of Related Structures:
2QTY - PubMed Abstract:
ADP-ribosylation is a reversible and covalent post-translational modification in which the attachment of ADP-ribose is catalyzed by ADP-ribosyltransferases and the removal of ADP-ribose is catalyzed by ADP-ribosylhydrolases. ADP-ribosylhydrolase 3 from mouse, consisting of 347 amino-acid residues, has been cloned, purified and crystallized. The three-dimensional structure has been resolved at a resolution of 1.8 A. The structure constitutes a compact all-alpha-helical protein with two Mg(2+) ions located in the active-site crevice. A structural comparison of mouse ADP-ribosylhydrolase 3 with its human orthologue shows a high degree of structural similarity. Furthermore, four prokaryotic proteins deposited in the PDB could be identified as being structurally related.
- ESRF, 6 Rue Jules Horowitz, F-38043 Grenoble CEDEX 09, France. muellerd@esrf.fr
Organizational Affiliation: