Structural basis for RanGTP independent entry of spliceosomal U snRNPs into the nucleus.
Wohlwend, D., Strasser, A., Dickmanns, A., Ficner, R.(2007) J Mol Biology 374: 1129-1138
- PubMed: 18028944
- DOI: https://doi.org/10.1016/j.jmb.2007.09.065
- Primary Citation of Related Structures:
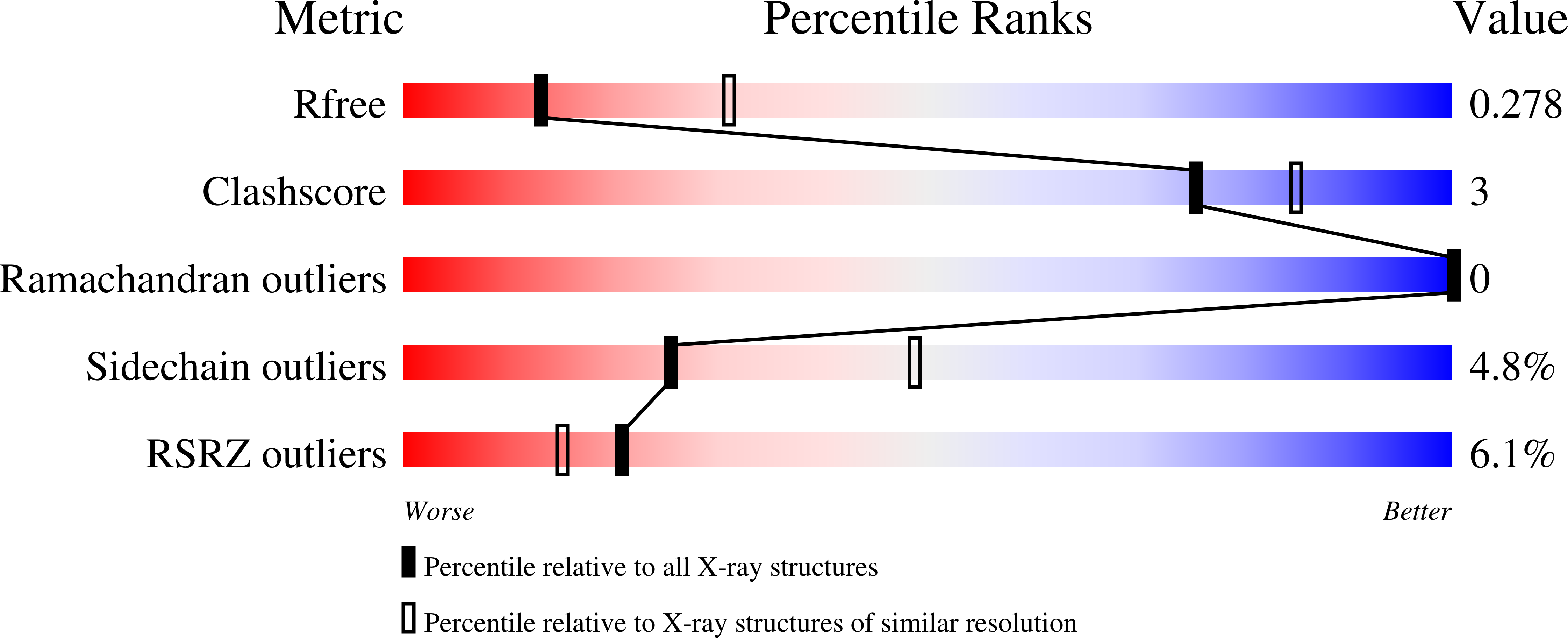
2QNA - PubMed Abstract:
The nuclear import of assembled spliceosomal subunits, the uridine-rich small nuclear ribonucleoprotein particles (U snRNPs), is mediated by a nuclear import receptor adaptor couple of importin beta (Imp beta) and snurportin1 (SPN1). In contrast to any other characterized active nuclear import, the Imp beta/SPN1/U snRNP complex does not require RanGTP for the terminal release from the nuclear basket of the nuclear pore complex (NPC). The crystal structure of Imp beta (127-876) in complex with the Imp beta-binding (IBB) domain of SPN1 (1-65) at 2.8-A resolution reveals that Imp beta adopts an open conformation, which is unique for a functional Imp beta/cargo complex, and rather surprisingly, it resembles the conformation of the Imp beta/RanGTP complex. As binding of RanGTP to Imp beta usually triggers the release of import complexes from the NPC, we propose that by already mimicking a conformation similar to Imp beta/RanGTP the independent dissociation of Imp beta/SPN1 from the nuclear basket is energetically aided.
- Abteilung für Molekulare Strukturbiologie, Institut für Mikrobiologie und Genetik and GZMB, Georg-August-Universität Göttingen, Justus-von-Liebig-Weg 11, D-37077 Göttingen, Germany.
Organizational Affiliation: