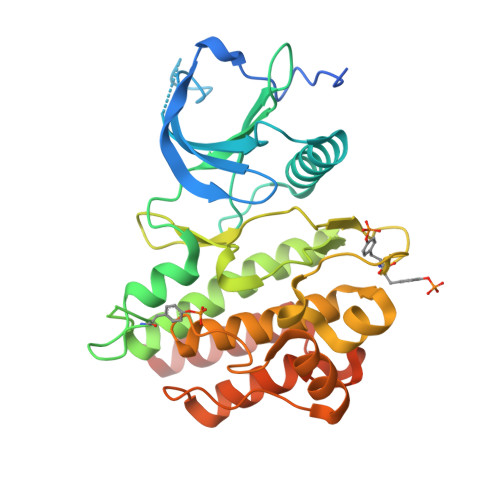
A molecular brake in the kinase hinge region regulates the activity of receptor tyrosine kinases.
Chen, H., Ma, J., Li, W., Eliseenkova, A.V., Xu, C., Neubert, T.A., Miller, W.T., Mohammadi, M.(2007) Mol Cell 27: 717-730
- PubMed: 17803937
- DOI: https://doi.org/10.1016/j.molcel.2007.06.028
- Primary Citation of Related Structures:
2PSQ, 2PVF, 2PVY, 2PWL, 2PY3, 2PZ5, 2PZP, 2PZR, 2Q0B - PubMed Abstract:
Activating mutations in the tyrosine kinase domain of receptor tyrosine kinases (RTKs) cause cancer and skeletal disorders. Comparison of the crystal structures of unphosphorylated and phosphorylated wild-type FGFR2 kinase domains with those of seven unphosphorylated pathogenic mutants reveals an autoinhibitory "molecular brake" mediated by a triad of residues in the kinase hinge region of all FGFRs. Structural analysis shows that many other RTKs, including PDGFRs, VEGFRs, KIT, CSF1R, FLT3, TEK, and TIE, are also subject to regulation by this brake. Pathogenic mutations activate FGFRs and other RTKs by disengaging the brake either directly or indirectly.
- Department of Pharmacology, New York University School of Medicine, New York, NY 10016, USA.
Organizational Affiliation: