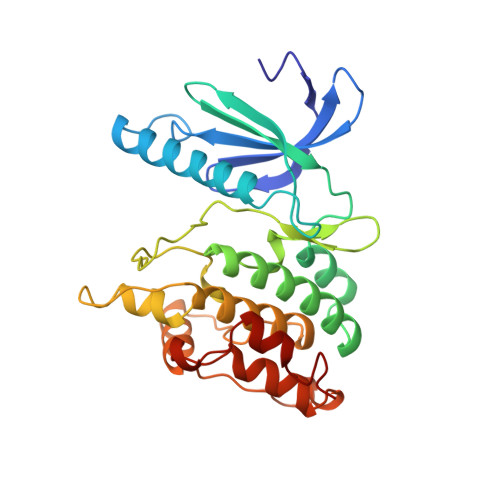
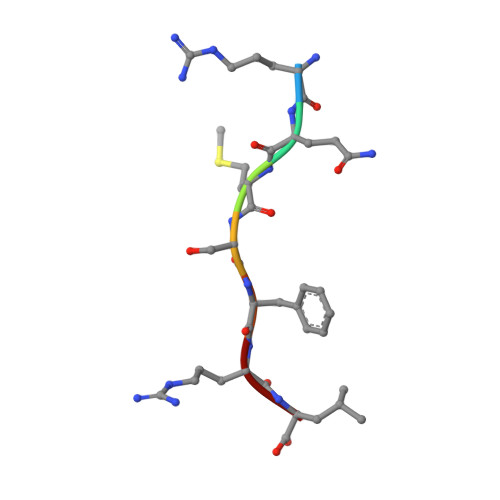
The crystal structure of a phosphorylase kinase peptide substrate complex: kinase substrate recognition.
Lowe, E.D., Noble, M.E., Skamnaki, V.T., Oikonomakos, N.G., Owen, D.J., Johnson, L.N.(1997) EMBO J 16: 6646-6658
- PubMed: 9362479
- DOI: https://doi.org/10.1093/emboj/16.22.6646
- Primary Citation of Related Structures:
2PHK - PubMed Abstract:
The structure of a truncated form of the gamma-subunit of phosphorylase kinase (PHKgammat) has been solved in a ternary complex with a non-hydrolysable ATP analogue (adenylyl imidodiphosphate, AMPPNP) and a heptapeptide substrate related in sequence to both the natural substrate and to the optimal peptide substrate. Kinetic characterization of the phosphotransfer reaction confirms the peptide to be a good substrate, and the structure allows identification of key features responsible for its high affinity. Unexpectedly, the substrate peptide forms a short anti-parallel beta-sheet with the kinase activation segment, the region which in other kinases plays an important role in regulation of enzyme activity. This anchoring of the main chain of the substrate peptide at a fixed distance from the gamma-phosphate of ATP explains the selectivity of PHK for serine/threonine over tyrosine as a substrate. The catalytic core of PHK exists as a dimer in crystals of the ternary complex, and the relevance of this phenomenon to its in vivo recognition of dimeric glycogen phosphorylase b is considered.
- Laboratory of Molecular Biophysics and Oxford Centre for Molecular Sciences, University of Oxford, Rex Richards Building, South Parks Road, Oxford OX1 3QU, UK.
Organizational Affiliation: