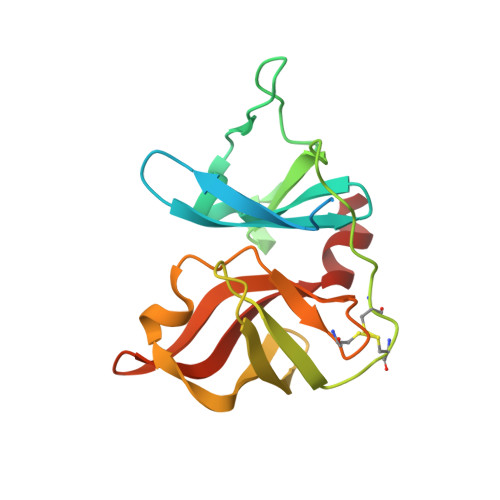
Inhibitors of hepatitis C virus NS3.4A protease. Effect of P4 capping groups on inhibitory potency and pharmacokinetics.
Perni, R.B., Chandorkar, G., Cottrell, K.M., Gates, C.A., Lin, C., Lin, K., Luong, Y.P., Maxwell, J.P., Murcko, M.A., Pitlik, J., Rao, G., Schairer, W.C., Van Drie, J., Wei, Y.(2007) Bioorg Med Chem Lett 17: 3406-3411
- PubMed: 17482818
- DOI: https://doi.org/10.1016/j.bmcl.2007.03.090
- Primary Citation of Related Structures:
2P59 - PubMed Abstract:
Reversible tetrapeptide-based compounds have been shown to effectively inhibit the hepatitis C virus NS3.4A protease. Inhibition of viral replicon RNA production in Huh-7 cells has also been demonstrated. We show herein that the inclusion of hydrogen bond donors on the P4 capping group of tetrapeptide-based inhibitors result in increased binding potency to the NS3.4A protease. The capping groups also impart significant effects on the pharmacokinetic profile of these inhibitors.
- Vertex Pharmaceuticals, Inc., 130 Waverly Street, Cambridge, MA 02139, USA. rperni@sirtrispharma.com
Organizational Affiliation: