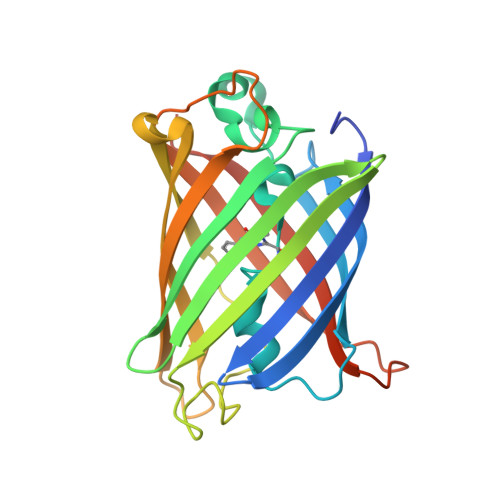
Design of a highly specific and noninvasive biosensor suitable for real-time in vivo imaging of mercury (II) uptake.
Chapleau, R.R., Blomberg, R., Ford, P.C., Sagermann, M.(2008) Protein Sci 17: 614-622
- PubMed: 18305194
- DOI: https://doi.org/10.1110/ps.073358908
- Primary Citation of Related Structures:
2OKW, 2OKY - PubMed Abstract:
Mercury is a ubiquitous pollutant that when absorbed is extremely toxic to a wide variety of biochemical processes. Mercury (II) is a strong, "invisible" poison that is rapidly absorbed by tissues of the intestinal tract, kidneys, and liver upon ingestion. In this study, a novel fluorescence-based biosensor is presented that allows for the direct monitoring of the uptake and distribution of the metal under noninvasive in vivo conditions. With the introduction of a cysteine residue at position 205, located in close proximity to the chromophore, the green fluorescent protein (GFP) from Aequorea victoria was converted into a highly specific biosensor for this metal ion. The mutant protein exhibits a dramatic absorbance and fluorescence change upon mercuration at neutral pH. Absorbance and fluorescence properties with respect to the metal concentration exhibit sigmoidal binding behavior with a detection limit in the low nanomolar range. Time-resolved binding studies indicate rapid subsecond binding of the metal to the protein. The crystal structures obtained of mutant eGFP205C indicate a possible access route of the metal into the core of the protein. To our knowledge, this engineered protein is a first example of a biosensor that allows for noninvasive and real-time imaging of mercury uptake in a living cell. A major advantage is that its expression can be genetically controlled in many organisms to enable unprecedented studies of tissue specific mercury uptake.
- Department of Chemistry and Biochemistry, University of California, Santa Barbara, Santa Barbara, California 93106-9510, USA.
Organizational Affiliation: