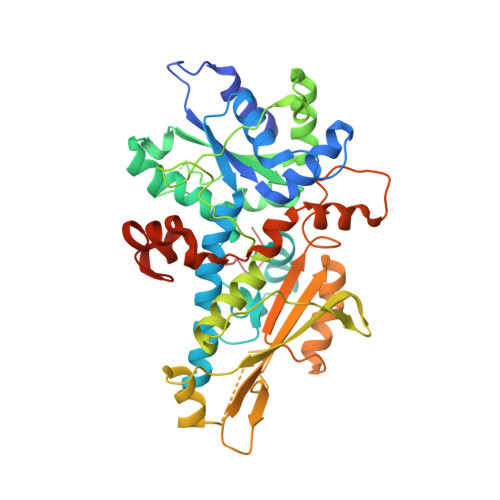
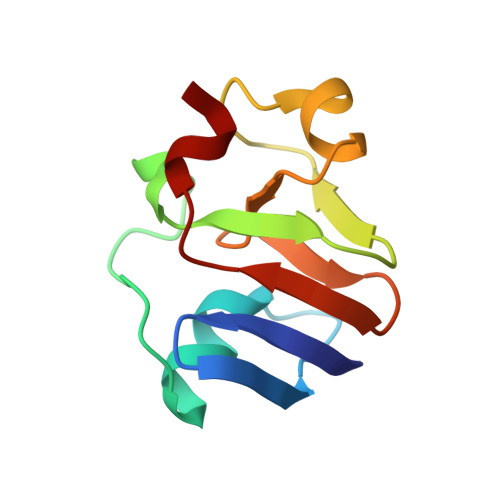
Structural Insight into the Complex of Ferredoxin and [FeFe] Hydrogenase from Chlamydomonas reinhardtii.
Rumpel, S., Siebel, J.F., Diallo, M., Fares, C., Reijerse, E.J., Lubitz, W.(2015) Chembiochem 16: 1663-1669
- PubMed: 26010059
- DOI: https://doi.org/10.1002/cbic.201500130
- Primary Citation of Related Structures:
2N0S - PubMed Abstract:
The transfer of photosynthetic electrons by the ferredoxin PetF to the [FeFe] hydrogenase HydA1 in the microalga Chlamydomonas reinhardtii is a key step in hydrogen production. Electron delivery requires a specific interaction between PetF and HydA1. However, because of the transient nature of the electron-transfer complex, a crystal structure remains elusive. Therefore, we performed protein-protein docking based on new experimental data from a solution NMR spectroscopy investigation of native and gallium-substituted PetF. This provides valuable information about residues crucial for complex formation and electron transfer. The derived complex model might help to pinpoint residue substitution targets for improved hydrogen production.
- Department for Biophysical Chemistry, Max-Planck-Institut für Chemische Energiekonversion, Stiftstrasse 34-36, 45470 Mülheim an der Ruhr (Germany). wolfgang.lubitz@cec.mpg.de.
Organizational Affiliation: