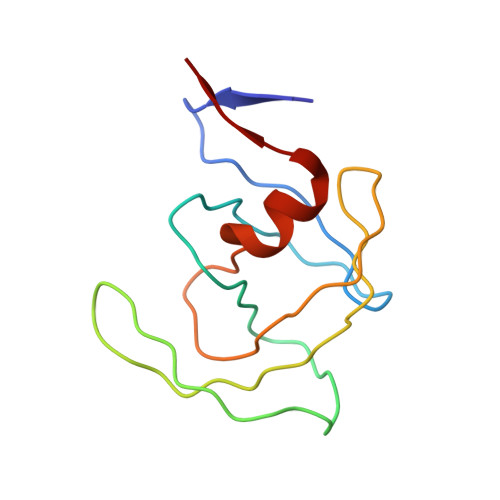
Crystal structure of human immunodeficiency virus (HIV) type 2 protease in complex with a reduced amide inhibitor and comparison with HIV-1 protease structures.
Tong, L., Pav, S., Pargellis, C., Do, F., Lamarre, D., Anderson, P.C.(1993) Proc Natl Acad Sci U S A 90: 8387-8391
- PubMed: 8378311
- DOI: https://doi.org/10.1073/pnas.90.18.8387
- Primary Citation of Related Structures:
2MIP - PubMed Abstract:
The crystal structure of HIV-2 protease in complex with a reduced amide inhibitor [BI-LA-398; Phe-Val-Phe-psi (CH2NH)-Leu-Glu-Ile-amide] has been determined at 2.2-A resolution and refined to a crystallographic R factor of 17.6%. The rms deviation from ideality in bond lengths is 0.018 A and in bond angles is 2.8 degrees. The largest structural differences between HIV-1 and HIV-2 proteases are located at residues 15-20, 34-40, and 65-73, away from the flap region and the substrate binding sites. The rms distance between equivalent C alpha atoms of HIV-1 and HIV-2 protease structures excluding these residues is 0.5 A. The shapes of the S1 and S2 pockets in the presence of this inhibitor are essentially unperturbed by the amino acid differences between HIV-1 and HIV-2 proteases. The interaction of the inhibitor with HIV-2 protease is similar to that observed in HIV-1 protease structures. The unprotected N terminus of the inhibitor interacts with the side chains of Asp-29 and Asp-30. The glutamate side chain of the inhibitor forms hydrogen bonds with the main-chain amido groups of residues 129 and 130.
- Department of Medicinal Chemistry, Boehringer Ingelheim Pharmaceutical, Inc., Ridgefield, CT 06877.
Organizational Affiliation: