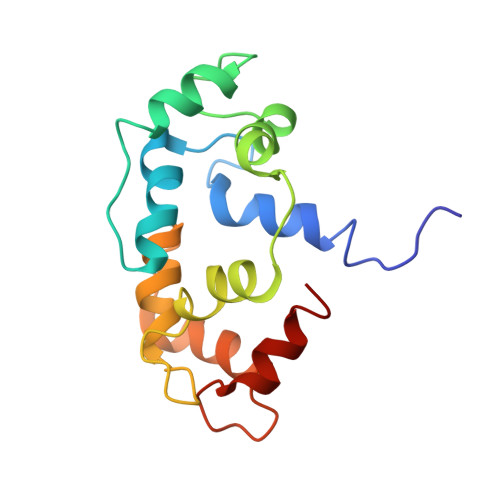
Solution structure of calmodulin bound to the target Peptide of endothelial nitric oxide synthase phosphorylated at thr495.
Piazza, M., Taiakina, V., Guillemette, S.R., Guillemette, J.G., Dieckmann, T.(2014) Biochemistry 53: 1241-1249
- PubMed: 24495081
- DOI: https://doi.org/10.1021/bi401466s
- Primary Citation of Related Structures:
2MG5 - PubMed Abstract:
Nitric oxide synthase (NOS) plays a major role in a number of key physiological and pathological processes, and it is important to understand how this enzyme is regulated. The small acidic calcium binding protein, calmodulin (CaM), is required to fully activate the enzyme. The exact mechanism of how CaM activates NOS is not fully understood at this time. Studies have shown CaM to act like a switch that causes a conformational change in NOS to allow for the transfer of an electron between the reductase and oxygenase domains through a process that is thought to be highly dynamic and at least in part controlled by several possible phosphorylation sites. We have determined the solution structure of CaM bound to a peptide that contains a phosphorylated threonine corresponding to Thr495 in full size endothelial NOS (eNOS) to investigate the structural and functional effects that the phosphorylation of this residue may have on nitric oxide production. Our biophysical studies show that phosphorylation of Thr495 introduces electrostatic repulsions between the target sequence and CaM as well as a diminished propensity for the peptide to form an α-helix. The calcium affinity of the CaM-target peptide complex is reduced because of phosphorylation, and this leads to weaker binding at low physiological calcium concentrations. This study provides an explanation for the reduced level of NO production by eNOS carrying a phosphorylated Thr495 residue.
- Department of Chemistry, University of Waterloo , Waterloo, Ontario N2L 3G1, Canada.
Organizational Affiliation: