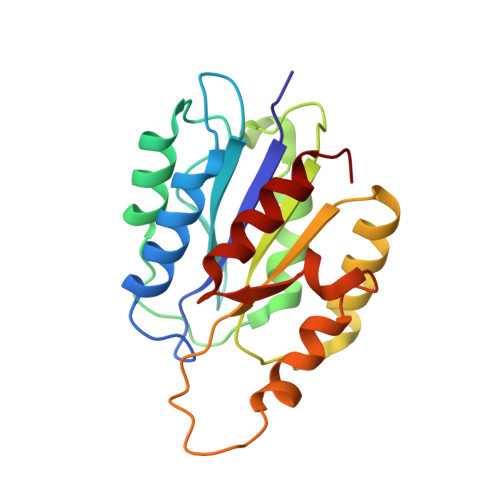
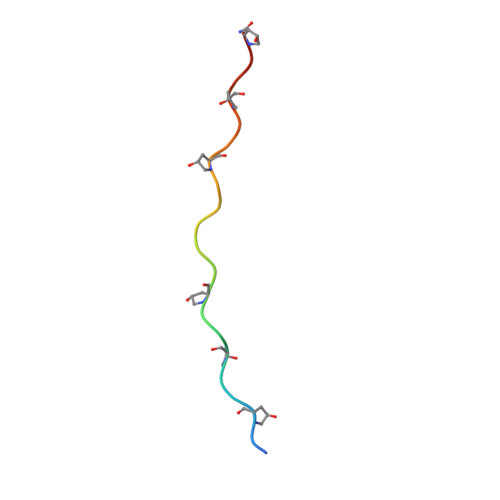
The Structure of Integrin alpha 1I Domain in Complex with a Collagen-mimetic Peptide.
Chin, Y.K., Headey, S.J., Mohanty, B., Patil, R., McEwan, P.A., Swarbrick, J.D., Mulhern, T.D., Emsley, J., Simpson, J.S., Scanlon, M.J.(2013) J Biological Chem 288: 36796-36809
- PubMed: 24187131
- DOI: https://doi.org/10.1074/jbc.M113.480251
- Primary Citation of Related Structures:
2M32 - PubMed Abstract:
We have determined the structure of the human integrin α1I domain bound to a triple-helical collagen peptide. The structure of the α1I-peptide complex was investigated using data from NMR, small angle x-ray scattering, and size exclusion chromatography that were used to generate and validate a model of the complex using the data-driven docking program, HADDOCK (High Ambiguity Driven Biomolecular Docking). The structure revealed that the α1I domain undergoes a major conformational change upon binding of the collagen peptide. This involves a large movement in the C-terminal helix of the αI domain that has been suggested to be the mechanism by which signals are propagated in the intact integrin receptor. The structure suggests a basis for the different binding selectivity observed for the α1I and α2I domains. Mutational data identify residues that contribute to the conformational change observed. Furthermore, small angle x-ray scattering data suggest that at low collagen peptide concentrations the complex exists in equilibrium between a 1:1 and 2:1 α1I-peptide complex.
- From Medicinal Chemistry, Monash Institute of Pharmaceutical Sciences and.
Organizational Affiliation: