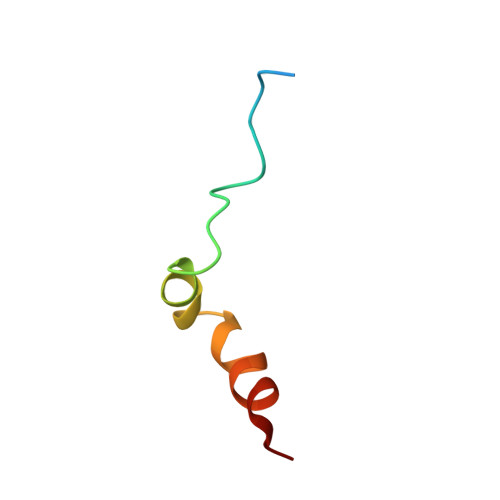
Structure, Sulfatide Binding Properties, and Inhibition of Platelet Aggregation by a Disabled-2 Protein-derived Peptide.
Xiao, S., Charonko, J.J., Fu, X., Salmanzadeh, A., Davalos, R.V., Vlachos, P.P., Finkielstein, C.V., Capelluto, D.G.(2012) J Biological Chem 287: 37691-37702
- PubMed: 22977233
- DOI: https://doi.org/10.1074/jbc.M112.385609
- Primary Citation of Related Structures:
2LSW - PubMed Abstract:
Disabled-2 (Dab2) targets membranes and triggers a wide range of biological events, including endocytosis and platelet aggregation. Dab2, through its phosphotyrosine-binding (PTB) domain, inhibits platelet aggregation by competing with fibrinogen for α(IIb)β(3) integrin receptor binding. We have recently shown that the N-terminal region, including the PTB domain (N-PTB), drives Dab2 to the platelet membrane surface by binding to sulfatides through two sulfatide-binding motifs, modulating the extent of platelet aggregation. The three-dimensional structure of a Dab2-derived peptide encompassing the sulfatide-binding motifs has been determined in dodecylphosphocholine micelles using NMR spectroscopy. Dab2 sulfatide-binding motif contains two helices when embedded in micelles, reversibly binds to sulfatides with moderate affinity, lies parallel to the micelle surface, and when added to a platelet mixture, reduces the number and size of sulfatide-induced aggregates. Overall, our findings identify and structurally characterize a minimal region in Dab2 that modulates platelet homotypic interactions, all of which provide the foundation for rational design of a new generation of anti-aggregatory low-molecular mass molecules for therapeutic purposes.
- Protein Signaling Domains Laboratory, Department of Biological Sciences, Virginia Tech, 1981 Kraft Dr., Rm. 2007, Blacksburg, VA 24061, USA.
Organizational Affiliation: