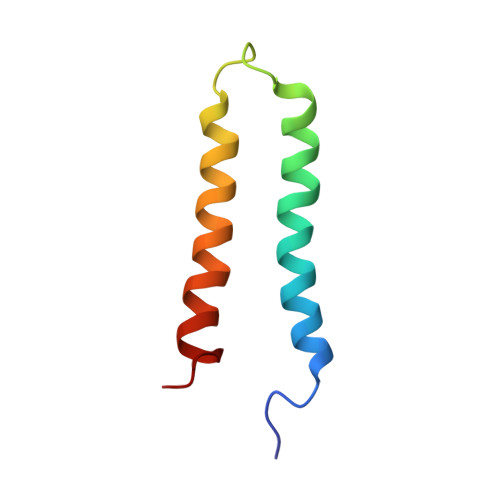
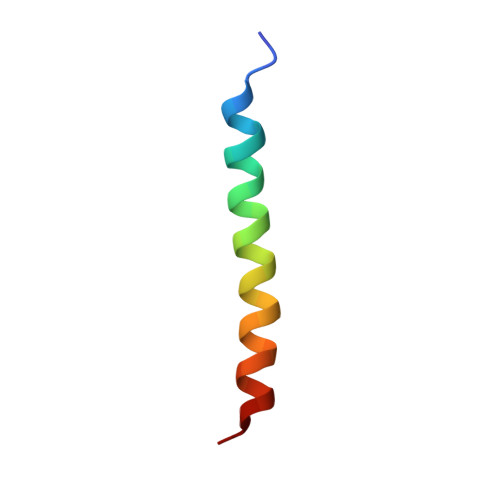
The structural basis for intramembrane assembly of an activating immunoreceptor complex.
Call, M.E., Wucherpfennig, K.W., Chou, J.J.(2010) Nat Immunol 11: 1023-1029
- PubMed: 20890284
- DOI: https://doi.org/10.1038/ni.1943
- Primary Citation of Related Structures:
2L34, 2L35 - PubMed Abstract:
Many receptors that activate cells of the immune system are multisubunit membrane protein complexes in which ligand recognition and signaling functions are contributed by separate protein modules. Receptors and signaling subunits assemble through contacts among basic and acidic residues in their transmembrane domains to form the functional complexes. Here we report the nuclear magnetic resonance (NMR) structure of the membrane-embedded, heterotrimeric assembly formed by association of the DAP12 signaling module with the natural killer (NK) cell-activating receptor NKG2C. The main intramembrane contact site is formed by a complex electrostatic network involving five hydrophilic transmembrane residues. Functional mutagenesis demonstrated that similar polar intramembrane motifs are also important for assembly of the NK cell-activating NKG2D-DAP10 complex and the T cell antigen receptor (TCR)-invariant signaling protein CD3 complex. This structural motif therefore lies at the core of the molecular organization of many activating immunoreceptors.
- Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, Boston, Massachusetts, USA.
Organizational Affiliation: