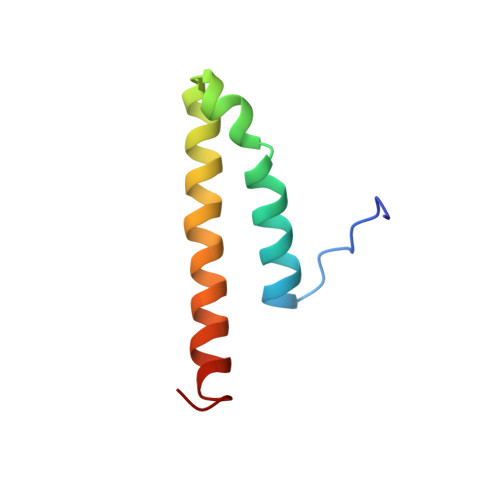
Human cyclin-dependent kinase 2-associated protein 1 (CDK2AP1) is dimeric in its disulfide-reduced state, with natively disordered N-terminal region.
Ertekin, A., Aramini, J.M., Rossi, P., Leonard, P.G., Janjua, H., Xiao, R., Maglaqui, M., Lee, H.W., Prestegard, J.H., Montelione, G.T.(2012) J Biological Chem 287: 16541-16549
- PubMed: 22427660
- DOI: https://doi.org/10.1074/jbc.M112.343863
- Primary Citation of Related Structures:
2KW6 - PubMed Abstract:
CDK2AP1 (cyclin-dependent kinase 2-associated protein 1), corresponding to the gene doc-1 (deleted in oral cancer 1), is a tumor suppressor protein. The doc-1 gene is absent or down-regulated in hamster oral cancer cells and in many other cancer cell types. The ubiquitously expressed CDK2AP1 protein is the only known specific inhibitor of CDK2, making it an important component of cell cycle regulation during G(1)-to-S phase transition. Here, we report the solution structure of CDK2AP1 by combined methods of solution state NMR and amide hydrogen/deuterium exchange measurements with mass spectrometry. The homodimeric structure of CDK2AP1 includes an intrinsically disordered 60-residue N-terminal region and a four-helix bundle dimeric structure with reduced Cys-105 in the C-terminal region. The Cys-105 residues are, however, poised for disulfide bond formation. CDK2AP1 is phosphorylated at a conserved Ser-46 site in the N-terminal "intrinsically disordered" region by IκB kinase ε.
- Center for Advanced Biotechnology and Medicine and Northeast Structural Genomics Consortium, Rutgers, The State University of New Jersey, Piscataway, New Jersey 08854, USA.
Organizational Affiliation: