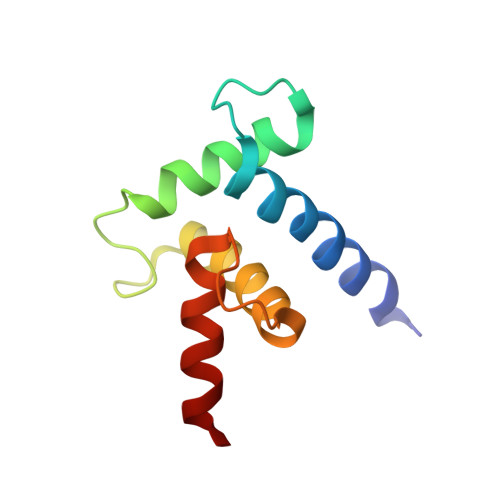
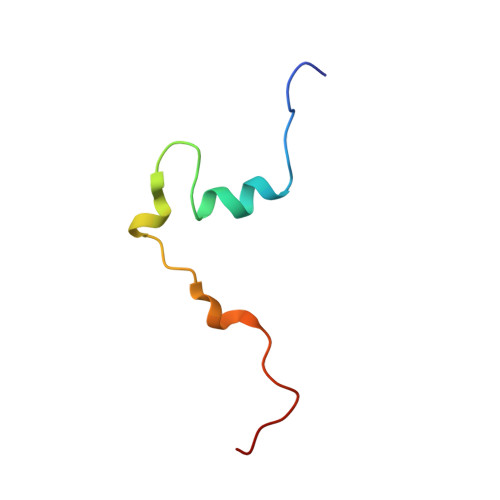
Structural basis for subversion of cellular control mechanisms by the adenoviral E1A oncoprotein.
Ferreon, J.C., Martinez-Yamout, M.A., Dyson, H.J., Wright, P.E.(2009) Proc Natl Acad Sci U S A 106: 13260-13265
- PubMed: 19651603
- DOI: https://doi.org/10.1073/pnas.0906770106
- Primary Citation of Related Structures:
2KJE - PubMed Abstract:
The adenovirus early region 1A (E1A) oncoprotein mediates cell transformation by deregulating host cellular processes and activating viral gene expression by recruitment of cellular proteins that include cyclic-AMP response element binding (CREB) binding protein (CBP)/p300 and the retinoblastoma protein (pRb). While E1A is capable of independent interaction with CBP/p300 or pRb, simultaneous binding of both proteins is required for maximal biological activity. To obtain insights into the mechanism by which E1A hijacks the cellular transcription machinery by competing with essential transcription factors for binding to CBP/p300, we have determined the structure of the complex between the transcriptional adaptor zinc finger-2 (TAZ2) domain of CBP and the conserved region-1 (CR1) domain of E1A. The E1A CR1 domain is unstructured in the free state and upon binding folds into a local helical structure mediated by an extensive network of intermolecular hydrophobic contacts. By NMR titrations, we show that E1A efficiently competes with the N-terminal transactivation domain of p53 for binding to TAZ2 and that pRb interacts with E1A at 2 independent sites located in CR1 and CR2. We show that pRb and the CBP TAZ2 domain can bind simultaneously to the CR1 site of E1A to form a ternary complex and propose a structural model for the pRb:E1A:CBP complex on the basis of published x-ray data for homologous binary complexes. These observations reveal the molecular basis by which E1A inhibits p53-mediated transcriptional activation and provide a rationale for the efficiency of cellular transformation by the adenoviral E1A oncoprotein.
- Department of Molecular Biology and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.
Organizational Affiliation: