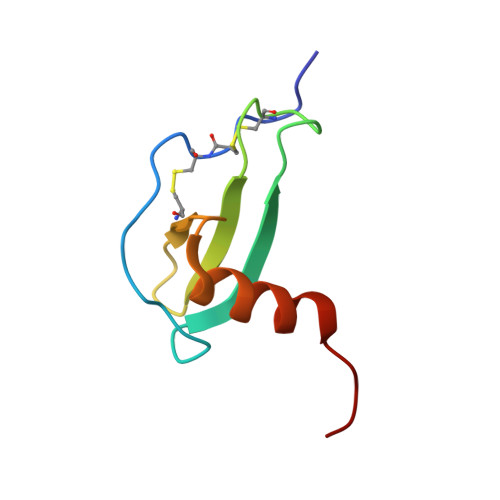
Human macrophage inflammatory protein 3alpha: protein and peptide nuclear magnetic resonance solution structures, dimerization, dynamics, and anti-infective properties.
Chan, D.I., Hunter, H.N., Tack, B.F., Vogel, H.J.(2008) Antimicrob Agents Chemother 52: 883-894
- PubMed: 18086840
- DOI: https://doi.org/10.1128/AAC.00805-07
- Primary Citation of Related Structures:
2JYO - PubMed Abstract:
Human macrophage inflammatory protein 3alpha (MIP-3alpha), also known as CCL20, is a 70-amino-acid chemokine which exclusively binds to chemokine receptor 6. In addition, the protein also has direct antimicrobial, antifungal, and antiviral activities. The solution structure of MIP-3alpha was solved by the use of two-dimensional homonuclear proton nuclear magnetic resonance (NMR). The structure reveals the characteristic chemokine fold, with three antiparallel beta strands followed by a C-terminal alpha helix. In contrast to the crystal structures of MIP-3alpha, the solution structure was found to be monomeric. Another difference between the NMR and crystal structures lies in the angle of the alpha helix with respect to the beta strands, which measure 69 and approximately 56.5 degrees in the two structures, respectively. NMR diffusion and pH titration studies revealed a distinct tendency for MIP-3alpha to form dimers at neutral pH and monomers at lower pH, dependent on the protonation state of His40. Molecular dynamics simulations of both the monomeric and the dimeric forms of MIP-3alpha supported the notion that the chemokine undergoes a change in helix angle upon dimerization and also highlighted the important hydrophobic and hydrogen bonding contacts made by His40 in the dimer interface. Moreover, a constrained N terminus and a smaller binding groove were observed in dimeric MIP-3alpha simulations, which could explain why monomeric MIP-3alpha may be more adept at receptor binding and activation. The solution structure of a synthetic peptide consisting of the last 20 residues of MIP-3alpha displayed a highly amphipathic alpha helix, reminiscent of various antimicrobial peptides. Antimicrobial assays with this peptide revealed strong and moderate bactericidal activities against Escherichia coli and Staphylococcus aureus, respectively. This confirms that the C-terminal alpha-helical region of MIP-3alpha plays a significant part in its broad anti-infective activity.
- Department of Biological Sciences, University of Calgary, 2500 University Drive NW, Calgary, Alberta T2N 1N4, Canada.
Organizational Affiliation: