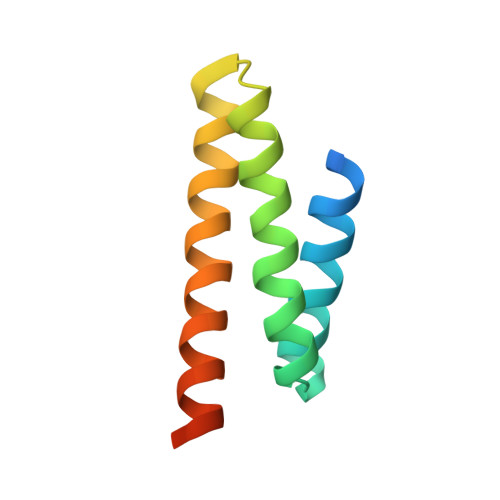
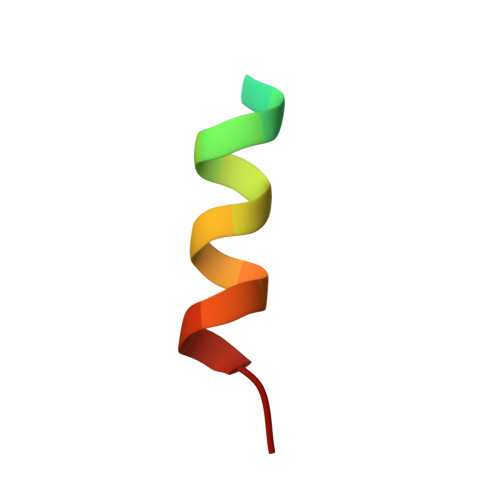
ESCRT-III recognition by VPS4 ATPases.
Stuchell-Brereton, M.D., Skalicky, J.J., Kieffer, C., Karren, M.A., Ghaffarian, S., Sundquist, W.I.(2007) Nature 449: 740-744
- PubMed: 17928862
- DOI: https://doi.org/10.1038/nature06172
- Primary Citation of Related Structures:
2JQ9, 2JQH, 2JQK - PubMed Abstract:
The ESCRT (endosomal sorting complex required for transport) pathway is required for terminal membrane fission events in several important biological processes, including endosomal intraluminal vesicle formation, HIV budding and cytokinesis. VPS4 ATPases perform a key function in this pathway by recognizing membrane-associated ESCRT-III assemblies and catalysing their disassembly, possibly in conjunction with membrane fission. Here we show that the microtubule interacting and transport (MIT) domains of human VPS4A and VPS4B bind conserved sequence motifs located at the carboxy termini of the CHMP1-3 class of ESCRT-III proteins. Structures of VPS4A MIT-CHMP1A and VPS4B MIT-CHMP2B complexes reveal that the C-terminal CHMP motif forms an amphipathic helix that binds in a groove between the last two helices of the tetratricopeptide-like repeat (TPR) of the VPS4 MIT domain, but in the opposite orientation to that of a canonical TPR interaction. Distinct pockets in the MIT domain bind three conserved leucine residues of the CHMP motif, and mutations that inhibit these interactions block VPS4 recruitment, impair endosomal protein sorting and relieve dominant-negative VPS4 inhibition of HIV budding. Thus, our studies reveal how the VPS4 ATPases recognize their CHMP substrates to facilitate the membrane fission events required for the release of viruses, endosomal vesicles and daughter cells.
- Department of Biochemistry, Room 4100, 15 N. Medical Drive East, University of Utah, Salt Lake City, Utah 84112-5650, USA.
Organizational Affiliation: