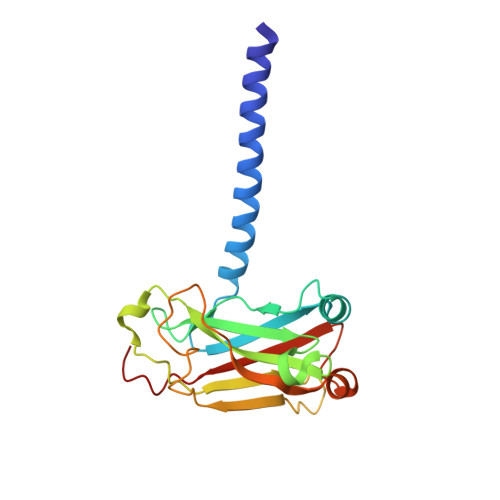
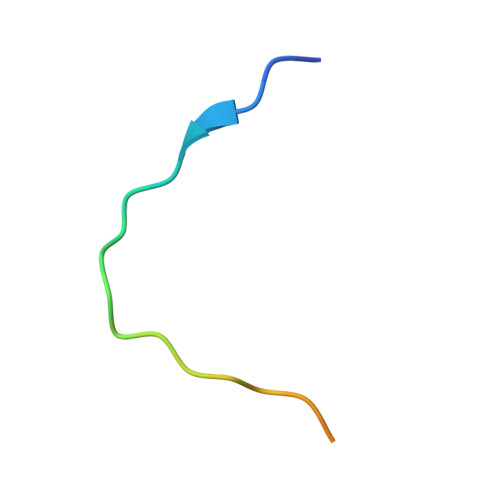
Key molecular contacts promote recognition of the BAFF receptor by TNF receptor-associated factor 3: implications for intracellular signaling regulation.
Ni, C.-Z., Oganesyan, G., Welsh, K., Zhu, X., Reed, J.C., Satterthwait, A.C., Cheng, G., Ely, K.R.(2004) J Immunol 173: 7394-7400
- PubMed: 15585864
- DOI: https://doi.org/10.4049/jimmunol.173.12.7394
- Primary Citation of Related Structures:
2GKW - PubMed Abstract:
B cell-activating factor belonging to the TNF family receptor (BAFF-R), a member of the TNFR superfamily, plays a role in autoimmunity after ligation with BAFF ligand (also called TALL-1, BLyS, THANK, or zTNF4). BAFF/BAFF-R interactions are critical for B cell regulation, and signaling from this ligand-receptor complex results in NF-kappaB activation. Most TNFRs transmit signals intracellularly by recruitment of adaptor proteins called TNFR-associated factors (TRAFs). However, BAFF-R binds only one TRAF adaptor, TRAF3, and this interaction negatively regulates activation of NF-kappaB. In this study, we report the crystal structure of a 24-residue fragment of the cytoplasmic portion of BAFF-R bound in complex with TRAF3. The recognition motif (162)PVPAT(166) in BAFF-R is accommodated in the same binding crevice on TRAF3 that binds two related TNFRs, CD40 and LTbetaR, but is presented in a completely different structural framework. This region of BAFF-R assumes an open conformation with two extended strands opposed at right angles that each make contacts with TRAF3. The recognition motif is located in the N-terminal arm and intermolecular contacts mediate TRAF recognition. In the C-terminal arm, key stabilizing contacts are made, including critical hydrogen bonds with Gln(379) in TRAF3 that define the molecular basis for selective binding of BAFF-R solely to this member of the TRAF family. A dynamic conformational adjustment of Tyr(377) in TRAF3 occurs forming a new intermolecular contact with BAFF-R that stabilizes the complex. The structure of the complex provides a molecular explanation for binding affinities and selective protein interactions in TNFR-TRAF interactions.
- Cancer Research Center, The Burnham Institute, La Jolla, CA 92037, USA.
Organizational Affiliation: