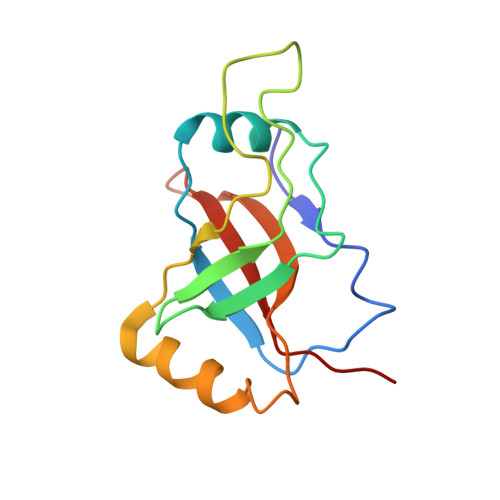
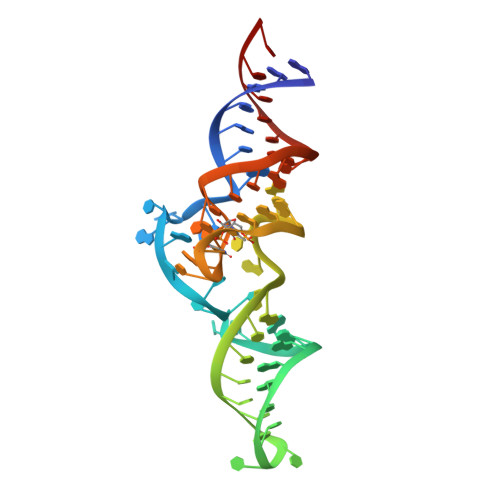
Structural basis for functional mimicry of long-variable-arm tRNA by transfer-messenger RNA.
Bessho, Y., Shibata, R., Sekine, S., Murayama, K., Higashijima, K., Hori-Takemoto, C., Shirouzu, M., Kuramitsu, S., Yokoyama, S.(2007) Proc Natl Acad Sci U S A 104: 8293-8298
- PubMed: 17488812
- DOI: https://doi.org/10.1073/pnas.0700402104
- Primary Citation of Related Structures:
1WJX, 2CZJ - PubMed Abstract:
tmRNA and small protein B (SmpB) are essential trans-translation system components. In the present study, we determined the crystal structure of SmpB in complex with the entire tRNA domain of the tmRNA from Thermus thermophilus. Overall, the ribonucleoprotein complex (tRNP) mimics a long-variable-arm tRNA (class II tRNA) in the canonical L-shaped tertiary structure. The tmRNA terminus corresponds to the acceptor and T arms, or the upper part, of tRNA. On the other hand, the SmpB protein simulates the lower part, the anticodon and D stems, of tRNA. Intriguingly, several amino acid residues collaborate with tmRNA bases to reproduce the canonical tRNA core layers. The linker helix of tmRNA had been considered to correspond to the anticodon stem, but the complex structure unambiguously shows that it corresponds to the tRNA variable arm. The tmRNA linker helix, as well as the long variable arm of class II tRNA, may occupy the gap between the large and small ribosomal subunits. This suggested how the tRNA domain is connected to the mRNA domain entering the mRNA channel. A loop of SmpB in the tRNP is likely to participate in the interaction with alanyl-tRNA synthetase, which may be the mechanism for the promotion of tmRNA alanylation by the SmpB protein. Therefore, the tRNP may simulate a tRNA, both structurally and functionally, with respect to aminoacylation and ribosome entry.
- Genomic Sciences Center, Yokohama Institute, RIKEN 1-7-22 Suehiro-cho, Tsurumi, Yokohama 230-0045, Japan.
Organizational Affiliation: