Construction of an expression system of canine milk lysozyme in the methylotrophic yeast Pichia pastoris
Akieda, D., Yasui, M., Aizawa, T., Yao, M., Watanabe, N., Tanaka, I., Demura, M., Kawano, K., Nitta, K.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Lysozyme C, milk isozyme | 129 | Canis lupus familiaris | Mutation(s): 0 EC: 3.2.1.17 | 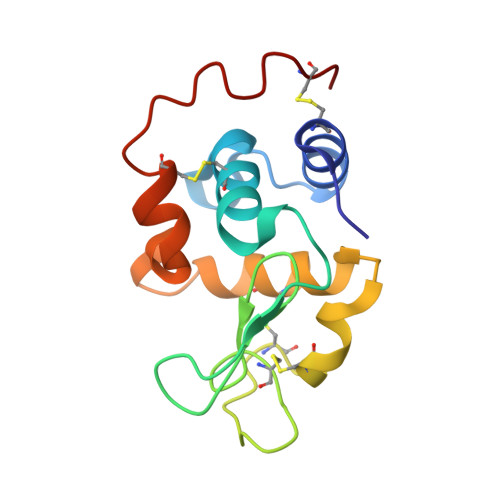 | |
UniProt | |||||
Find proteins for P81708 (Canis lupus familiaris) Explore P81708 Go to UniProtKB: P81708 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P81708 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| SO4 Query on SO4 | C [auth A], D [auth A], E [auth B], F [auth B] | SULFATE ION O4 S QAOWNCQODCNURD-UHFFFAOYSA-L |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 31.048 | α = 90 |
| b = 31.048 | β = 90 |
| c = 199.757 | γ = 120 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| MOSFLM | data reduction |
| CCP4 | data scaling |
| XFIT | data reduction |