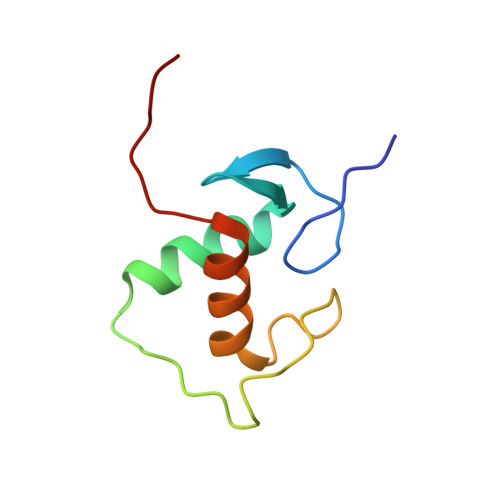
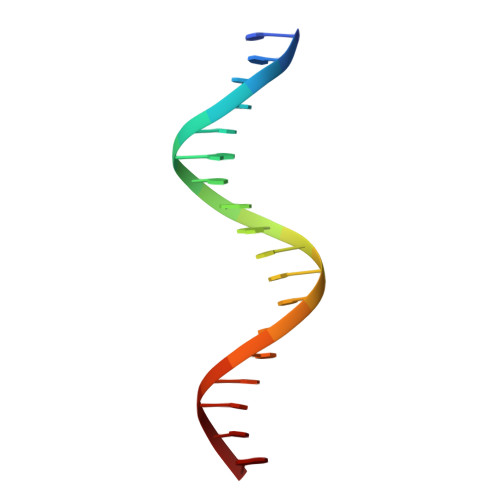
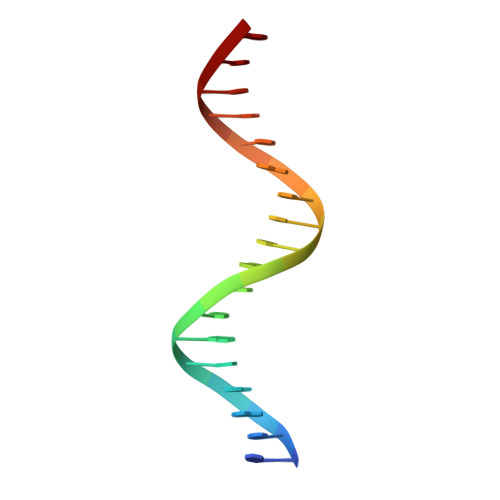
Structure of the Progesterone Receptor-Deoxyribonucleic Acid Complex: Novel Interactions Required for Binding to Half-Site Response Elements.
Roemer, S.C., Donham, D.C., Sherman, L., Pon, V.H., Edwards, D.P., Churchill, M.E.A.(2006) Mol Endocrinol 20: 3042
- PubMed: 16931575
- DOI: https://doi.org/10.1210/me.2005-0511
- Primary Citation of Related Structures:
2C7A - PubMed Abstract:
The DNA binding domain (DBD) of nuclear hormone receptors contains a highly conserved globular domain and a less conserved carboxyl-terminal extension (CTE). Despite previous observations that the CTEs of some classes of nuclear receptors are structured and interact with DNA outside of the hexanucleotide hormone response element (HRE), there has been no evidence for such a CTE among the steroid receptors. We have determined the structure of the progesterone receptor (PR)-DBD-CTE DNA complex at a resolution of 2.5 A, which revealed binding of the CTE to the minor groove flanking the HREs. Alanine substitutions of the interacting CTE residues reduced affinity for inverted repeat HREs separated by three nucleotides, and essentially abrogated binding to a single HRE. A highly compressed minor groove of the trinucleotide spacer and a novel dimerization interface were also observed. A PR binding site selection experiment revealed sequence preferences in the trinucleotide spacer and flanking DNA. These results, taken together, support the notion that sequences outside of the HREs influence the DNA binding affinity and specificity of steroid receptors.
- Program in Molecular Biology, Department of Pharmacology, University of Colorado at Denver and Health Sciences Center, Aurora, Colorado 80045, USA.
Organizational Affiliation: