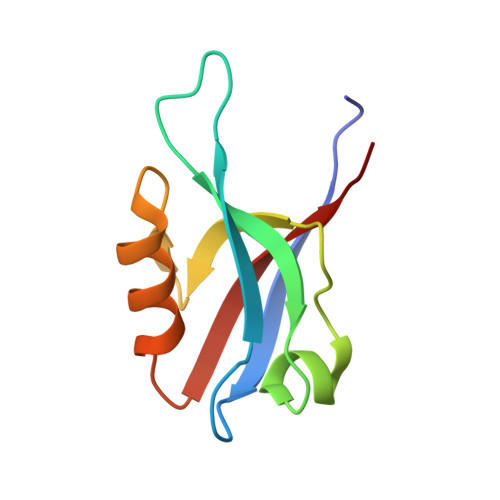
Solution structure and backbone dynamics of the AF-6 PDZ domain/Bcr peptide complex.
Chen, Q., Niu, X., Xu, Y., Wu, J., Shi, Y.(2007) Protein Sci 16: 1053-1062
- PubMed: 17473018
- DOI: https://doi.org/10.1110/ps.062440607
- Primary Citation of Related Structures:
2AIN - PubMed Abstract:
The human AF-6, a scaffold protein between cell membrane-associated proteins and the actin cytoskeleton, plays an important role in special cell-cell junctions and signal transduction. It can be phosphorylated by the protein kinase Bcr, which allows efficient binding of the C terminus of Bcr to the PDZ domain of AF-6 and consequently enhances the binding affinity of AF-6 to Ras. Formation of the AF-6, Bcr, and Ras ternary complex results in down-regulation of the Ras-mediated signal transduction pathway. To better understand the molecular basis for the recognition of the AF-6 PDZ domain and Bcr, we solve the solution structure of the AF-6 PDZ domain complexed with the C-terminal peptide of Bcr and explore the interactions between them in detail. Compared with previously reported structures, the complex exhibits a noncanonical binding mode of PDZ/peptide. Owing to the distinct residues involved in the AF-6 PDZ domain and Bcr peptide interaction, the interaction mode does not adapt to the existing classification rules that have been put forward, based on the ligand or the PDZ domain specificity. Furthermore, the PDZ domain of AF-6 can bind to the C terminus of Bcr efficiently after phosphorylation of AF-6 by the Bcr kinase. The phosphorylation may induce a conformational change of AF-6, which makes the binding surface on the PDZ domain accessible to Bcr for efficient binding. This study not only characterizes the structural details of the AF-6 PDZ/Bcr peptide complex, but also provides a potential target for future drug design and disease therapy.
- Hefei National Laboratory for Physical Sciences at the Microscale and School of Life Science, University of Science and Technology of China, Hefei, Anhui 230026, China.
Organizational Affiliation: