Thiophene-Based Diamidine Forms a "Super" at Binding Minor Groove Agent
Mallena, S., Lee, M.P.H., Bailly, C., Neidle, S., Kumar, A., Boykin, D.W., Wilson, W.D.(2004) J Am Chem Soc 142: 13659
- PubMed: 15493923
- DOI: https://doi.org/10.1021/ja048175m
- Primary Citation of Related Structures:
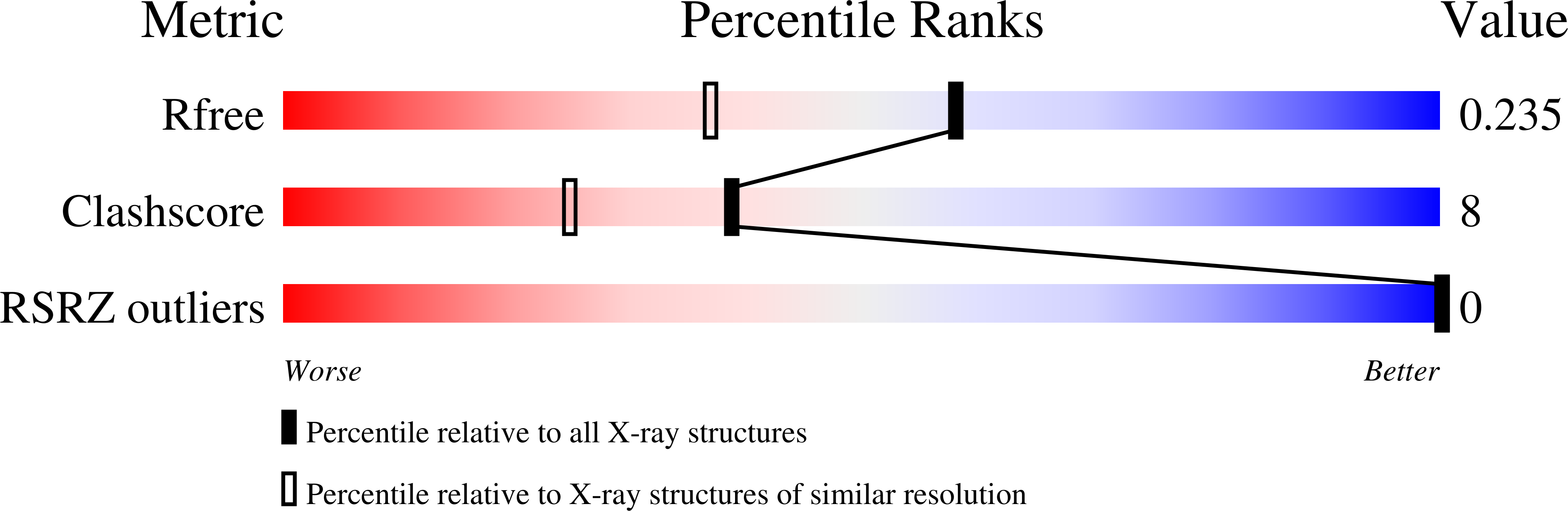
1VZK - PubMed Abstract:
The DNA minor groove is the interaction site for many enzymes and transcription control proteins and as a result, development of compounds that target the minor groove is an active research area. In an effort to develop biologically active minor groove agents, we are preparing and exploring the DNA interactions of a systematic set of diamidine derivatives with a powerful array of methods including DNase I footprinting, biosensor-SPR methods, and X-ray crystallography. Surprisingly, conversion of the parent phenyl-furan-phenyl diamidine to a phenyl-thiophene-benzimidazole derivative yields a compound with over 10-fold-increased affinity for the minor groove at AT sequences. Single conversion of the furan to a thiophene or a phenyl to benzimidazole does not cause a similar increase in affinity. X-ray results indicate a small bond angle difference between the C-S-C angle of thiophene and the C-O-C angle of furan that, when amplified out to the terminal amidines of the benzimidazole compounds, yields a very significant difference in the positions of the amidines and their DNA interaction strength.
- Department of Chemistry, Georgia State University, P.O. Box 4098, Atlanta, Georgia 30302-4098, USA.
Organizational Affiliation: