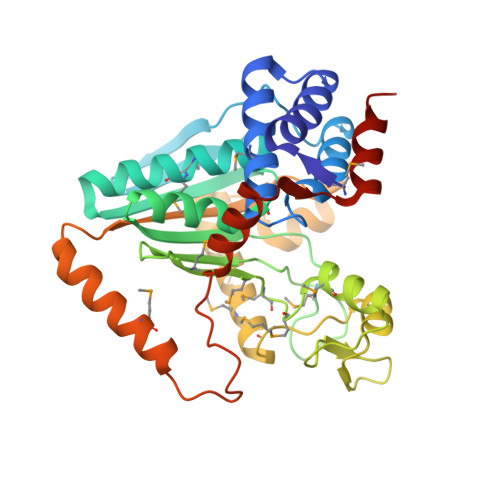
A Novel NAD-binding Protein Revealed by the Crystal Structure of 2,3-Diketo-L-gulonate Reductase (YiaK).
Forouhar, F., Lee, I., Benach, J., Kulkarni, K., Xiao, R., Acton, T.B., Montelione, G.T., Tong, L.(2004) J Biological Chem 279: 13148-13155
- PubMed: 14718529
- DOI: https://doi.org/10.1074/jbc.M313580200
- Primary Citation of Related Structures:
1NXU, 1S20 - PubMed Abstract:
Escherichia coli YiaK catalyzes the reduction of 2,3-diketo-L-gulonate in the presence of NADH. It belongs to a large family of oxidoreductases that is conserved in archaea, bacteria, and eukaryotes but shows no sequence homology to other proteins. We report here the crystal structures at up to 2.0-A resolution of YiaK alone and in complex with NAD-tartrate. YiaK has a new polypeptide backbone fold and a novel mode of recognizing the NAD cofactor. In addition, NAD is bound in an unusual conformation, at the interface of a dimer of the enzyme. The crystallographic analysis unexpectedly revealed the binding of tartrate in the active site. Enzyme kinetics studies confirm that tartrate and the related D-malate are inhibitors of YiaK. In contrast to most other enzymes where substrate binding produces a more closed conformation, the binding of NAD-tartrate to YiaK produces a more open active site. The free enzyme conformation is incompatible with NAD binding. His(44) is likely the catalytic residue of the enzyme.
- Department of Biological Sciences, Northeast Structural Genomics Consortium, Columbia University, New York, NY 10027, USA.
Organizational Affiliation: