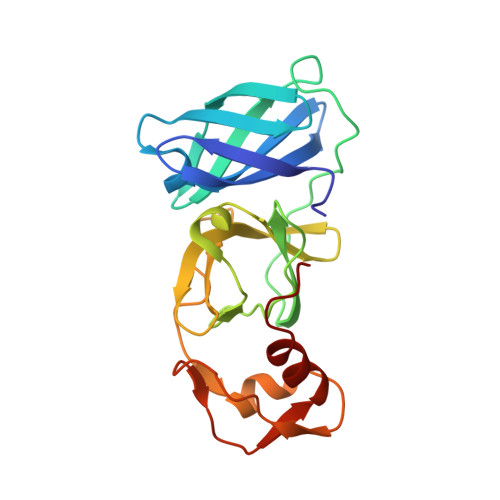
Structure of Arterivirus nsp4: the smallest chymotrypsin-like proteinase with an alpha/beta C-terminal extension and alternate conformations of the oxyanion hole
Barrette-Ng, I.H., Ng, K.K.-S., Mark, B.L., van Aken, D., Cherney, M.M., Garen, C., Kolodenko, Y., Gorbalenya, A.E., Snijder, E.J., James, M.N.G.(2002) J Biological Chem 277: 39960-39966
- PubMed: 12163505
- DOI: https://doi.org/10.1074/jbc.M206978200
- Primary Citation of Related Structures:
1MBM - PubMed Abstract:
Arteriviruses are enveloped, positive-stranded RNA viruses and include pathogens of major economic concern to the swine- and horse-breeding industries. The arterivirus replicase gene encodes two large precursor polyproteins that are processed by the viral main proteinase nonstructural protein 4 (nsp4). The three-dimensional structure of the 21-kDa nsp4 from the arterivirus prototype equine arteritis virus has been determined to 2.0 A resolution. Nsp4 adopts the smallest known chymotrypsin-like fold with a canonical catalytic triad of Ser-120, His-39, and Asp-65, as well as a novel alpha/beta C-terminal extension domain that may play a role in mediating protein-protein interactions. In different copies of nsp4 in the asymmetric unit, the oxyanion hole adopts either a collapsed inactive conformation or the standard active conformation, which may be a novel way of regulating proteolytic activity.
- Canadian Institutes for Health Research Group in Protein Structure and Function, Department of Biochemistry, University of Alberta, Edmonton, Alberta T6G 2H7, Canada.
Organizational Affiliation: