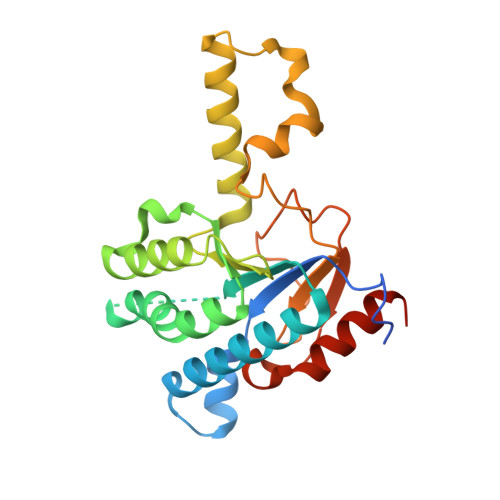
Crystal structure of cis-prenyl chain elongating enzyme, undecaprenyl diphosphate synthase.
Fujihashi, M., Zhang, Y.W., Higuchi, Y., Li, X.Y., Koyama, T., Miki, K.(2001) Proc Natl Acad Sci U S A 98: 4337-4342
- PubMed: 11287651
- DOI: https://doi.org/10.1073/pnas.071514398
- Primary Citation of Related Structures:
1F75 - PubMed Abstract:
Undecaprenyl diphosphate synthase (UPS) catalyzes the cis-prenyl chain elongation onto trans, trans-farnesyl diphosphate (FPP) to produce undecaprenyl diphosphate (UPP), which is indispensable for the biosynthesis of bacterial cell walls. We report here the crystal structure of UPS as the only three-dimensional structure among cis-prenyl chain elongating enzymes. The structure is classified into a protein fold family and is completely different from the so-called "isoprenoid synthase fold" that is believed to be a common structure for the enzymes relating to isoprenoid biosynthesis. Conserved amino acid residues among cis-prenyl chain elongating enzymes are located around a large hydrophobic cleft in the UPS structure. A structural P-loop motif, which frequently appears in the various kinds of phosphate binding site, is found at the entrance of this cleft. The catalytic site is determined on the basis of these structural features, from which a possible reaction mechanism is proposed.
- Department of Chemistry, Graduate School of Science, Kyoto University, Sakyo-ku, Kyoto 606-8502, Japan.
Organizational Affiliation: