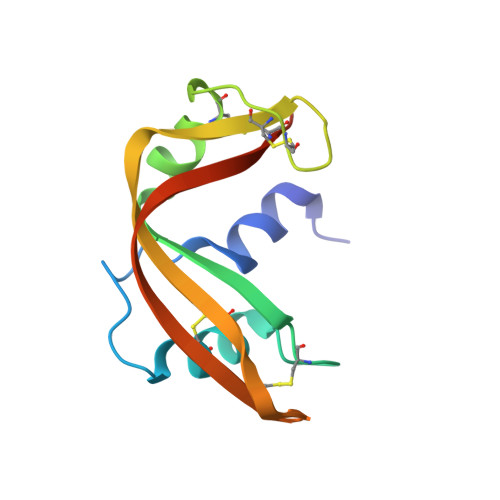
Structural investigation of catalytically modified F120L and F120Y semisynthetic ribonucleases.
deMel, V.S., Doscher, M.S., Glinn, M.A., Martin, P.D., Ram, M.L., Edwards, B.F.(1994) Protein Sci 3: 39-50
- PubMed: 8142897
- DOI: https://doi.org/10.1002/pro.5560030106
- Primary Citation of Related Structures:
1SSA, 1SSB - PubMed Abstract:
The structures of two catalytically modified semisynthetic RNases obtained by replacing phenylalanine 120 with leucine and tyrosine have been determined and refined at a resolution of 2.0 A (R = 0.161 and 0.184, respectively). These structures have been compared with the refined 1.8-A structure (R = 0.204) of the fully active phenylalanine-containing enzyme (Martin PD, Doscher MS, Edwards BFP, 1987, J Biol Chem 262:15930-15938) and with the catalytically defective D121A (2.0 A, R = 0.172) and D121N (2.0 A, R = 0.186) analogs (deMel VSJ, Martin PD, Doscher MS, Edwards BFP, 1992, J Biol Chem 267:247-256). The movement away from the active site of the loop containing residues 65-72 is seen in all three catalytically defective analogs--F120L, D121A, and D121N--but not in the fully active (or hyperactive) F120Y. The insertion of the phenolic hydroxyl of Tyr 120 into a hydrogen-bonding network involving the hydroxyl group of Ser 123 and a water molecule in F120Y is the likely basis for the hyperactivity toward uridine 2',3'-cyclic phosphate previously found for this analog (Hodges RS, Merrifield RB, 1974, Int J Pept Protein Res 6:397-405) as well as the threefold increase in KM for cytidine 2',3'-cyclic phosphate found for this analog by ourselves.
- Department of Biochemistry, Wayne State University School of Medicine, Detroit, Michigan 48201.
Organizational Affiliation: