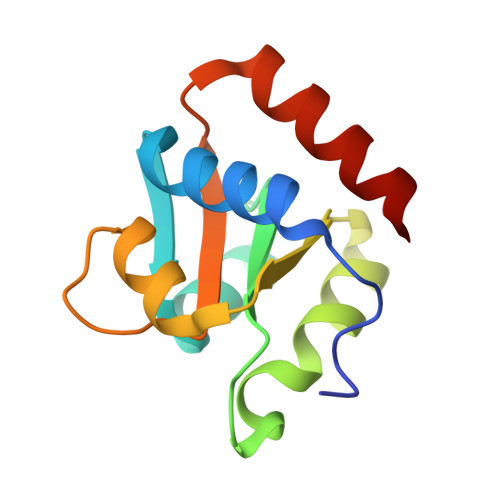
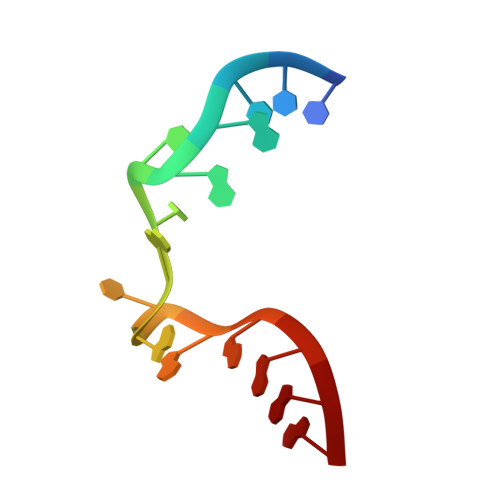
Structure of Protein L7Ae Bound to a K-Turn Derived from an Archaeal Box H/ACA sRNA at 1.8 A Resolution.
Hamma, T., Ferre-D'Amare, A.(2004) Structure 12: 893-903
- PubMed: 15130481
- DOI: https://doi.org/10.1016/j.str.2004.03.015
- Primary Citation of Related Structures:
1SDS - PubMed Abstract:
The archaeal RNA binding protein L7Ae and its eukaryotic homolog 15.5 kDa/Snu13 recognize K-turns. This structural motif is canonically comprised of two stems (one with tandem A.G base pairs, the other with Watson-Crick pairs) linked by an asymmetric internal loop. L7Ae recognizes conventional K-turns in ribosomal and box C/D RNAs but also binds specifically to some box H/ACA RNAs at terminal stem loops. These have the A.G paired stem, but lack the Watson-Crick stem. The structure of Methanococcus jannaschii L7Ae bound to a symmetric duplex RNA without Watson-Crick stems demonstrates how a binding site for this component of diverse ribonucleoprotein complexes can be constructed with only the A.G stem and the loop. The RNA adopts a functional conformation with the aid of a base triple and tight binding of divalent cations. Comparison with the 15.5 kDa/Snu13-RNA complex structure suggests why the eukaryotic homolog does not recognize terminal stem loop L7Ae binding sites.
- Division of Basic Sciences, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue North, Seattle, WA 98109 USA.
Organizational Affiliation: