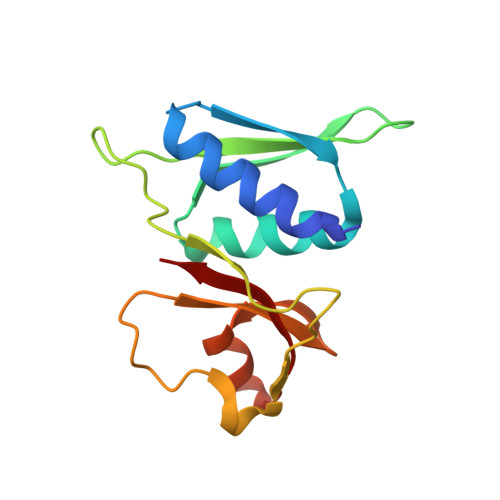
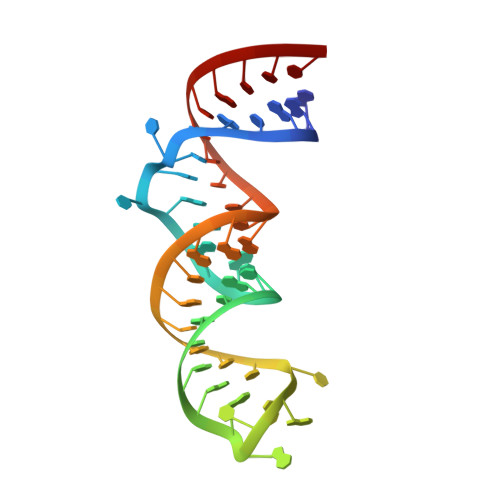
The structure of a ribosomal protein S8/spc operon mRNA complex.
Merianos, H.J., Wang, J., Moore, P.B.(2004) RNA 10: 954-964
- PubMed: 15146079
- DOI: https://doi.org/10.1261/rna.7030704
- Primary Citation of Related Structures:
1S03 - PubMed Abstract:
In bacteria, translation of all the ribosomal protein cistrons in the spc operon mRNA is repressed by the binding of the product of one of them, S8, to an internal sequence at the 5' end of the L5 cistron. The way in which the first two genes of the spc operon are regulated, retroregulation, is mechanistically distinct from translational repression by S8 of the genes from L5 onward. A 2.8 A resolution crystal structure has been obtained of Escherichia coli S8 bound to this site. Despite sequence differences, the structure of this complex is almost identical to that of the S8/helix 21 complex seen in the small ribosomal subunit, consistent with the hypothesis that autogenous regulation of ribosomal protein synthesis results from conformational similarities between mRNAs and rRNAs. S8 binding must repress the translation of its own mRNA by inhibiting the formation of a ribosomal initiation complex at the start of the L5 cistron.
- Department of Chemistry, Yale University, New Haven, Connecticut 06520, USA.
Organizational Affiliation: