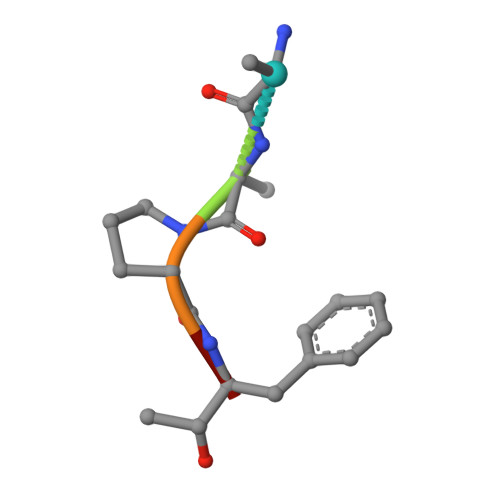
The 2.2 A crystal structure of human chymase in complex with succinyl-Ala-Ala-Pro-Phe-chloromethylketone: structural explanation for its dipeptidyl carboxypeptidase specificity.
Pereira, P.J., Wang, Z.M., Rubin, H., Huber, R., Bode, W., Schechter, N.M., Strobl, S.(1999) J Mol Biology 286: 163-173
- PubMed: 9931257
- DOI: https://doi.org/10.1006/jmbi.1998.2462
- Primary Citation of Related Structures:
1PJP - PubMed Abstract:
Human chymase (HC) is a chymotrypsin-like serine proteinase expressed by mast cells. The 2.2 A crystal structure of HC complexed to the peptidyl inhibitor, succinyl-Ala-Ala-Pro-Phe-chloromethylketone (CMK), was solved and refined to a crystallographic R-factor of 18.4 %. The HC structure exhibits the typical folding pattern of a chymotrypsin-like serine proteinase, and shows particularly similarity to rat chymase 2 (rat mast cell proteinase II) and human cathepsin G. The peptidyl-CMK inhibitor is covalently bound to the active-site residues Ser195 and His57; the peptidyl moiety juxtaposes the S1 entrance frame segment 214-217 by forming a short antiparallel beta-sheet. HC is a highly efficient angiotensin-converting enzyme. Modeling of the chymase-angiotensin I interaction guided by the geometry of the bound chloromethylketone inhibitor indicates that the extended substrate binding site contains features that may generate the dipeptidyl carboxypeptidase-like activity needed for efficient cleavage and activation of the hormone. The C-terminal carboxylate group of angiotensin I docked into the active-site cleft, with the last two residues extending beyond the active site, is perfectly localized to make a favorable hydrogen bond and salt bridge with the amide nitrogen of the Lys40-Phe41 peptide bond and with the epsilon-ammonium group of the Lys40 side-chain. This amide positioning is unique to the chymase-related proteinases, and only chymases from primates possess a Lys residue at position 40. Thus, the structure conveniently explains the preferred conversion of angiotensin I to angiotensin II by human chymase.
- Abteilung für Strukturforschung, Max-Planck-Institut für Biochemie, Am Klopferspitz 18a, Martinsried, D-82152, Germany.
Organizational Affiliation: