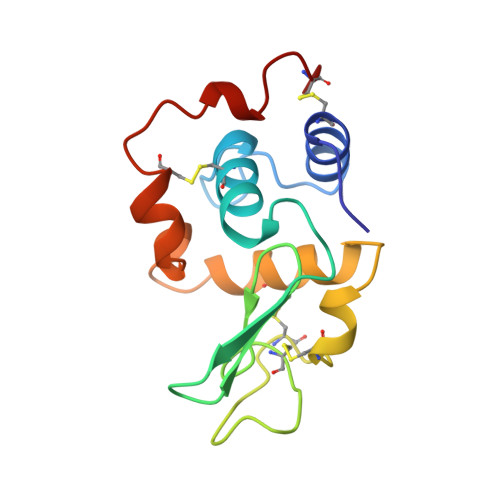
The structure, stability, and folding process of amyloidogenic mutant human lysozyme.
Funahashi, J., Takano, K., Ogasahara, K., Yamagata, Y., Yutani, K.(1996) J Biochem 120: 1216-1223
- PubMed: 9010773
- DOI: https://doi.org/10.1093/oxfordjournals.jbchem.a021544
- Primary Citation of Related Structures:
1OUA - PubMed Abstract:
The physicochemical properties of an amyloidogenic mutant human lysozyme (Ile56Thr) were examined in order to elucidate the mechanism of amyloid formation. The crystal structure of the mutant protein was the same as the wild-type structure, except that the hydroxyl group of the introduced Thr56 formed a hydrogen bond with a water molecule in the interior of the protein. The other physicochemical properties of the mutant protein in the native state were not different from those of the wild-type protein. However, the equilibrium and kinetic stabilities of the mutant protein were remarkably decreased due to the introduction of a polar residue (Thr) in the interior of the molecule. It can be concluded that the amyloid formation of the mutant human lysozyme is due to a tendency to favor (partly or/and completely) denatured structures.
- Institute for Protein Research, Osaka University.
Organizational Affiliation: