Structural basis for antibiotic recognition by the TipA-class of multidrug-resistance transcriptional regulators
Kahmann, J.D., Sass, H.J., Allan, M.G., Seto, H., Thompson, C.J., Grzesiek, S.(2003) EMBO J 22: 1824-1834
- PubMed: 12682015
- DOI: https://doi.org/10.1093/emboj/cdg181
- Primary Citation of Related Structures:
1NY9 - PubMed Abstract:
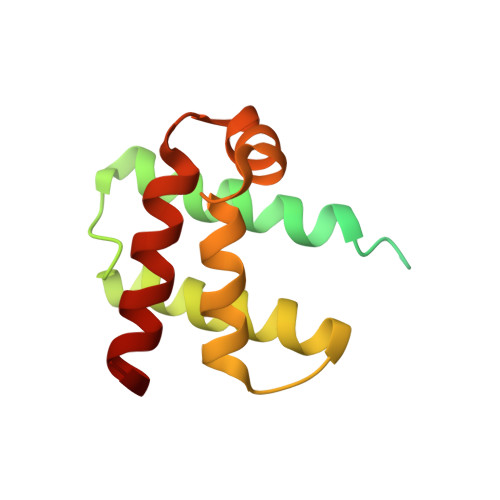
The TipAL protein, a bacterial transcriptional regulator of the MerR family, is activated by numerous cyclic thiopeptide antibiotics. Its C-terminal drug-binding domain, TipAS, defines a subfamily of broadly distributed bacterial proteins including Mta, a central regulator of multidrug resistance in Bacillus subtilis. The structure of apo TipAS, solved by solution NMR [Brookhaven Protein Data Bank entry 1NY9], is composed of a globin-like alpha-helical fold with a deep surface cleft and an unfolded N-terminal region. Antibiotics bind within the cleft at a position that is close to the corresponding heme pocket in myo- and hemoglobin, and induce folding of the N-terminus. Thus the classical globin fold is well adapted not only for accommodating its canonical cofactors, heme and other tetrapyrroles, but also for the recognition of a variety of antibiotics where ligand binding leads to transcriptional activation and drug resistance.
- Division of Structural Biology, Biozentrum der Universität Basel, Klingelbergstrasse 70, CH-4056 Basel, Switzerland.
Organizational Affiliation: