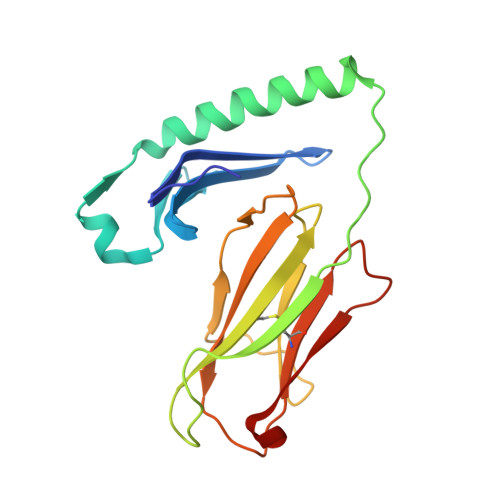
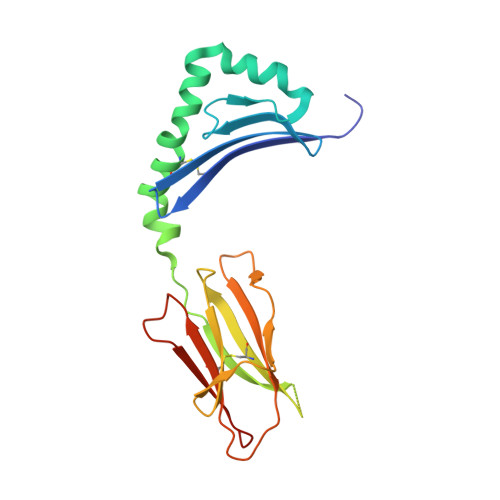
Crystal structure of MHC class II I-Ab in complex with a human CLIP peptide: Prediction of an I-Ab peptide-binding motif
Zhu, Y., Rudensky, A.Y., Teyton, A.L., Wilson, I.A.(2003) J Mol Biology 326: 1157-1174
- PubMed: 12589760
- DOI: https://doi.org/10.1016/s0022-2836(02)01437-7
- Primary Citation of Related Structures:
1MUJ - PubMed Abstract:
Association between the class II major histocompatibility complex (MHC) and the class II invariant chain-associated peptide (CLIP) occurs naturally as an intermediate step in the MHC class II processing pathway. Here, we report the crystal structure of the murine class II MHC molecule I-A(b) in complex with human CLIP at 2.15A resolution. The structure of I-A(b) accounts, via the peptide-binding groove's unique physicochemistry, for the distinct peptide repertoire bound by this allele. CLIP adopts a similar conformation to peptides bound by other I-A alleles, reinforcing the notion that CLIP is presented as a conventional peptide antigen. When compared to the related HLA-DR3/CLIP complex structure, the CLIP peptide displays a slightly different conformation and distinct interaction pattern with residues in I-A(b). In addition, after examining the published sequences of peptides presented by I-A(b), we discuss the possibility of predicting peptide alignment in the I-A(b) binding groove using a simple scoring matrix.
- Department of Molecular Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.
Organizational Affiliation: