Conformational variability of a picornavirus capsid: pH-dependent structural changes of Mengo virus related to its host receptor attachment site and disassembly.
Kim, S., Boege, U., Krishnaswamy, S., Minor, I., Smith, T.J., Luo, M., Scraba, D.G., Rossmann, M.G.(1990) Virology 175: 176-190
- PubMed: 2155508
- DOI: https://doi.org/10.1016/0042-6822(90)90198-z
- Primary Citation of Related Structures:
1MEC - PubMed Abstract:
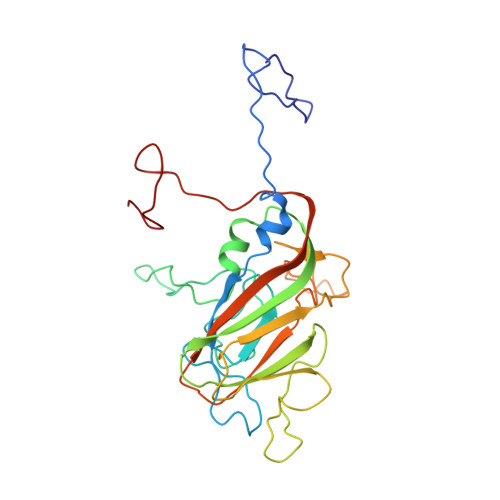
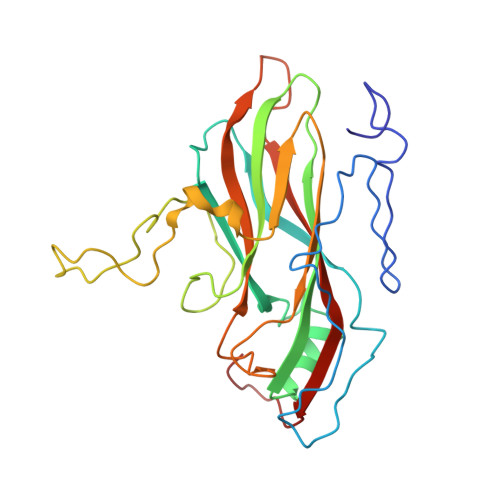
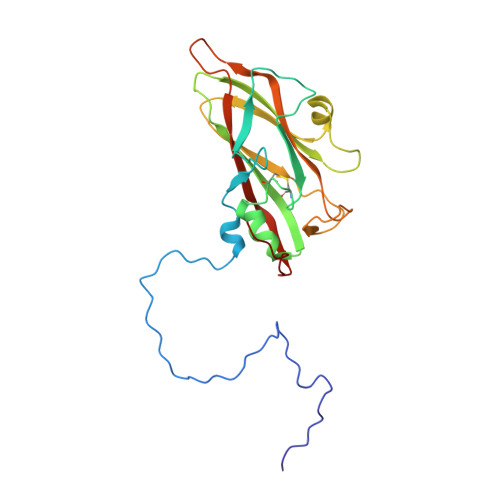
The structure of Mengo virus had been determined from crystals grown in the presence of 100 mM phosphate buffer at pH 7.4. It is shown that Mengo virus is poorly infectious at the phosphate concentration similar to that in which it was crystallized. Maximal infectivity is achieved at 10 mM phosphate or less in physiological saline. The phosphate effect is ameliorated when the pH is lowered to 4.6. Although it has not been possible to study the crystal structure of the virus at low phosphate concentrations, it is shown that increasing the Cl- concentration at pH 6.2 or decreasing the pH to 4.6 causes substantial conformational changes confined to the "pit," a deep surface depression. These structural changes involve a movement of the "FMDV loop" (GH loop) in VP1, an ordering of the "VP3 loop" (GH loop in VP3) between 3176 and 3182, the displacement of a bound phosphate near the "FMDV loop" (GH loop in VP1), and movement of the carboxy terminus of VP2. The changes in conformation are correlated with the dissociation of the virion into pentamers at pH 6.2 and 150 mM Cl-. The localization of the conformational changes and the correlated role of the phosphate in controlling infectivity support the hypothesis that the "pit" is the receptor attachment site.
- Department of Biological Sciences, Purdue University, West Lafayette, Indiana 47907.
Organizational Affiliation: